当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Perfluoro-Nickelacyclopentane Formation from Tetrafluoroethylene: Effects of Ancillary Ligand Bite Angle
Organometallics ( IF 2.5 ) Pub Date : 2023-11-17 , DOI: 10.1021/acs.organomet.3c00362 Luana L. T. N. Porto 1 , Behnaz Ghaffari 1 , Jeffrey S. Ovens 2 , Russell P. Hughes 3 , R. Tom Baker 1
Organometallics ( IF 2.5 ) Pub Date : 2023-11-17 , DOI: 10.1021/acs.organomet.3c00362 Luana L. T. N. Porto 1 , Behnaz Ghaffari 1 , Jeffrey S. Ovens 2 , Russell P. Hughes 3 , R. Tom Baker 1
Affiliation
|
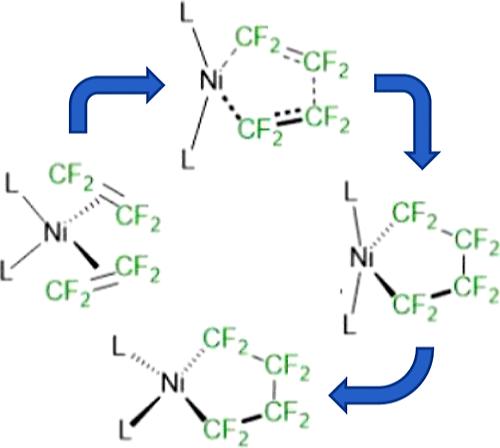
Metallacyclopentanes are important reaction intermediates for catalytic processes, such as selective ethylene oligomerization and diene cyclooligomerization. Reactions of fluoroalkenes with low-valent first row transition metal complexes tend to form stable fluorometallacyclopentane products. DFT studies conducted herein indicate that conversion of three-coordinate Ni(η2-C2F4)L2 complexes (L2 = 2 monodentate or one bidentate chelate) to perfluorometallacycle products Ni[κ2-(CF2)4-]L2 occurs preferentially via a four-coordinate intermediate Ni(η2-C2F4)2L2; an alternative three-coordinate pathway via Ni(η2-C2F4)2L occurs only when L is extremely bulky. The transition structure for cyclization requires a dramatic increase in the L–Ni–L angle, which is perpendicular to the C–C bond-forming plane and initially affords a kinetic metallacycle intermediate resembling a trigonal bipyramidal geometry with one equatorial vacant site. The donor solvent, or intramolecular O-donor association with this intermediate, provides a low-energy pathway to the formation of the more stable square-planar metallacycle product. For bidentate chelate ligands, differential bite angles affect both coordination of a second C2F4 ligand and the cyclization step. For small-bite-angle chelates, the cyclization transition state energies are higher relative to monodentate analogues. An experimental investigation of wide bite-angle bis(phosphine) ligands such as DPEphos and Bisbi showed the formation of additional nickelacyclopentane products with the coordination of a single P center to each Ni [DPEphos = bis(2-diphenylphosphino-phenyl) ether; Bisbi = 2,2′-bis(diphenylphosphino-methyl)-1,1′-biphenyl].
中文翻译:

四氟乙烯形成全氟镍环戊烷的机理:辅助配体咬角的影响
金属环戊烷是催化过程的重要反应中间体,例如选择性乙烯低聚和二烯环低聚。氟代烯烃与低价第一排过渡金属络合物的反应倾向于形成稳定的氟代金属环戊烷产物。本文进行的 DFT 研究表明,三配位 Ni(η 2 -C 2 F 4 )L 2配合物(L 2 = 2 个单齿或一个双齿螯合物)转化为全氟金属环产物 Ni[κ 2 -(CF 2 ) 4 -] L 2优先通过四配位中间体Ni(η 2 -C 2 F 4 ) 2 L 2发生;仅当 L 体积极大时,才会出现通过 Ni(η 2 -C 2 F 4 ) 2 L的替代三配位路径。环化的过渡结构需要大幅增加 L-Ni-L 角度,该角度垂直于 C-C 键形成平面,并最初提供一种类似于三角双锥几何形状的动力学金属环中间体,具有一个赤道空位。供体溶剂或与该中间体的分子内O-供体缔合提供了形成更稳定的方形平面金属环产物的低能量途径。对于双齿螯合配体,不同的咬角影响第二个C 2 F 4配体的配位和环化步骤。对于小咬角螯合物,环化过渡态能量相对于单齿类似物更高。对宽咬角双(膦)配体(例如 DPEphos 和 Bisbi)的实验研究表明,通过单个 P 中心与每个 Ni 的配位,形成了额外的环戊烷镍产物 [DPEphos = 双(2-二苯基膦基苯基)醚;Bisbi = 2,2'-双(二苯基膦基-甲基)-1,1'-联苯]。
更新日期:2023-11-17
中文翻译:

四氟乙烯形成全氟镍环戊烷的机理:辅助配体咬角的影响
金属环戊烷是催化过程的重要反应中间体,例如选择性乙烯低聚和二烯环低聚。氟代烯烃与低价第一排过渡金属络合物的反应倾向于形成稳定的氟代金属环戊烷产物。本文进行的 DFT 研究表明,三配位 Ni(η 2 -C 2 F 4 )L 2配合物(L 2 = 2 个单齿或一个双齿螯合物)转化为全氟金属环产物 Ni[κ 2 -(CF 2 ) 4 -] L 2优先通过四配位中间体Ni(η 2 -C 2 F 4 ) 2 L 2发生;仅当 L 体积极大时,才会出现通过 Ni(η 2 -C 2 F 4 ) 2 L的替代三配位路径。环化的过渡结构需要大幅增加 L-Ni-L 角度,该角度垂直于 C-C 键形成平面,并最初提供一种类似于三角双锥几何形状的动力学金属环中间体,具有一个赤道空位。供体溶剂或与该中间体的分子内O-供体缔合提供了形成更稳定的方形平面金属环产物的低能量途径。对于双齿螯合配体,不同的咬角影响第二个C 2 F 4配体的配位和环化步骤。对于小咬角螯合物,环化过渡态能量相对于单齿类似物更高。对宽咬角双(膦)配体(例如 DPEphos 和 Bisbi)的实验研究表明,通过单个 P 中心与每个 Ni 的配位,形成了额外的环戊烷镍产物 [DPEphos = 双(2-二苯基膦基苯基)醚;Bisbi = 2,2'-双(二苯基膦基-甲基)-1,1'-联苯]。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号