当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of the Imidazo[1,2-a]pyrazine Derivative A4 as a Potential Influenza Virus Nucleoprotein Inhibitor
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2023-11-17 , DOI: 10.1021/acsptsci.3c00174 Ping Li 1, 2 , Han Ju 1, 3 , Yihong Xing 3 , Fabao Zhao 1 , Varada Anirudhan 4 , Ruikun Du 2 , Qinghua Cui 2 , Xinyong Liu 1 , Lijun Rong 4 , Peng Zhan 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2023-11-17 , DOI: 10.1021/acsptsci.3c00174 Ping Li 1, 2 , Han Ju 1, 3 , Yihong Xing 3 , Fabao Zhao 1 , Varada Anirudhan 4 , Ruikun Du 2 , Qinghua Cui 2 , Xinyong Liu 1 , Lijun Rong 4 , Peng Zhan 1
Affiliation
|
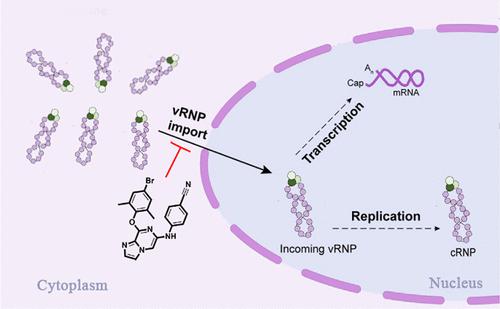
Influenza A viruses (IAVs) have gradually developed resistance to FDA-approved drugs, which increases the need to discover novel antivirals with new mechanisms of action. Here, we used a phenotypic screening strategy and discovered that the imidazo[1,2-a]pyrazine derivative A4 demonstrates potent and broad-spectrum anti-influenza activity, especially for the oseltamivir-resistant H1N1/pdm09 strain. Indirect immunofluorescence assays revealed that A4 induces clustering of the viral nucleoprotein (NP) and prevents its nuclear accumulation. Furthermore, upon conducting binding analyses between A4 and the influenza NP using surface plasmon resonance assays and molecular docking simulations, we were able to confirm that A4 binds directly to the viral NP. Additionally, A4 exhibits high human plasma metabolic stability (remaining120 min > 90%, T1/2 = 990 min) and moderate inhibitory effects on CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 as well as low acute toxicity in Kunming mice. Overall, this study provides valuable insights and lays the groundwork for future efforts in medicinal chemistry to identify effective drugs against influenza.
中文翻译:

鉴定咪唑[1,2-a]吡嗪衍生物 A4 作为潜在的流感病毒核蛋白抑制剂
甲型流感病毒 (IAV) 逐渐对 FDA 批准的药物产生耐药性,这增加了发现具有新作用机制的新型抗病毒药物的需求。在这里,我们使用了表型筛选策略,发现咪唑[1,2-a]吡嗪衍生物 A4 表现出有效和广谱的抗流感活性,特别是对于奥司他韦耐药的 H1N1/pdm09 菌株。间接免疫荧光测定显示 A4 诱导病毒核蛋白 (NP) 聚集并阻止其核积累。此外,通过使用表面等离子体共振测定和分子对接模拟对 A4 和流感 NP 之间的结合分析,我们能够确认 A4 直接与病毒 NP 结合。此外,A4 表现出较高的人血浆代谢稳定性 (剩余120 min > 90%,T1/2 = 990 min) 和对 CYP1A2 、 CYP2C9 、 CYP2C19 、 CYP2D6 和 CYP3A4 的中等抑制作用,以及对昆明小鼠的低急性毒性。总的来说,这项研究提供了有价值的见解,并为未来药物化学确定有效流感药物的努力奠定了基础。
更新日期:2023-11-17
中文翻译:

鉴定咪唑[1,2-a]吡嗪衍生物 A4 作为潜在的流感病毒核蛋白抑制剂
甲型流感病毒 (IAV) 逐渐对 FDA 批准的药物产生耐药性,这增加了发现具有新作用机制的新型抗病毒药物的需求。在这里,我们使用了表型筛选策略,发现咪唑[1,2-a]吡嗪衍生物 A4 表现出有效和广谱的抗流感活性,特别是对于奥司他韦耐药的 H1N1/pdm09 菌株。间接免疫荧光测定显示 A4 诱导病毒核蛋白 (NP) 聚集并阻止其核积累。此外,通过使用表面等离子体共振测定和分子对接模拟对 A4 和流感 NP 之间的结合分析,我们能够确认 A4 直接与病毒 NP 结合。此外,A4 表现出较高的人血浆代谢稳定性 (剩余120 min > 90%,T1/2 = 990 min) 和对 CYP1A2 、 CYP2C9 、 CYP2C19 、 CYP2D6 和 CYP3A4 的中等抑制作用,以及对昆明小鼠的低急性毒性。总的来说,这项研究提供了有价值的见解,并为未来药物化学确定有效流感药物的努力奠定了基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号