Pharmacological Research ( IF 9.1 ) Pub Date : 2023-11-18 , DOI: 10.1016/j.phrs.2023.106990 Zi-Hui Wang 1 , Jin Li 2 , Qian Liu 1 , Jian-Chang Qian 3 , Qing-Qing Li 3 , Qing-Yu Wang 2 , Lv-Tao Zeng 2 , Si-Jia Li 2 , Xin Gao 2 , Jia-Xin Pan 2 , Xu-Fan Gao 4 , Kun Wu 5 , Guo-Xin Hu 3 , Tomoo Iwakuma 6 , Jian-Ping Cai 1
|
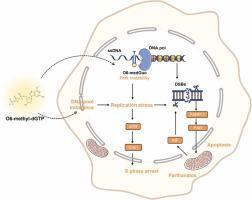
Resistance to temozolomide (TMZ), the frontline chemotherapeutic agent for glioblastoma (GBM), has emerged as a formidable obstacle, underscoring the imperative to identify alternative therapeutic strategies to improve patient outcomes. In this study, we comprehensively evaluated a novel agent, O6-methyl-2′-deoxyguanosine-5′-triphosphate (O6-methyl-dGTP) for its anti-GBM activity both in vitro and in vivo. Notably, O6-methyl-dGTP exhibited pronounced cytotoxicity against GBM cells, including those resistant to TMZ and overexpressing O6-methylguanine-DNA methyltransferase (MGMT). Mechanistic investigations revealed that O6-methyl-dGTP could be incorporated into genomic DNA, disrupting nucleotide pools balance, and inducing replication stress, resulting in S-phase arrest and DNA damage. The compound exerted its anti-tumor properties through the activation of AIF-mediated apoptosis and the parthanatos pathway. In vivo studies using U251 and Ln229 cell xenografts supported the robust tumor-inhibitory capacity of O6-methyl-dGTP. In an orthotopic transplantation model with U87MG cells, O6-methyl-dGTP showcased marginally superior tumor-suppressive activity compared to TMZ. In summary, our research, for the first time, underscores the potential of O6-methyl-dGTP as an effective candidate against GBM, laying a robust scientific groundwork for its potential clinical adoption in GBM treatment regimens.
中文翻译:

修饰的核苷 O6-甲基-2'-脱氧鸟苷-5'-三磷酸以不依赖半胱天冬酶的方式表现出抗胶质母细胞瘤活性
胶质母细胞瘤 (GBM) 的一线化疗药物替莫唑胺 (TMZ) 的耐药性已成为一个巨大的障碍,这凸显了寻找替代治疗策略以改善患者预后的必要性。在这项研究中,我们全面评估了一种新型药物O6-甲基-2'-脱氧鸟苷-5'-三磷酸(O6-甲基-dGTP)的体外和体内抗GBM活性。值得注意的是,O6-甲基-dGTP 对 GBM 细胞表现出明显的细胞毒性,包括对 TMZ 耐药和过度表达 O6-甲基鸟嘌呤-DNA 甲基转移酶 (MGMT) 的细胞。机制研究表明,O6-甲基-dGTP 可以整合到基因组 DNA 中,破坏核苷酸池平衡,并诱导复制应激,导致 S 期停滞和 DNA 损伤。该化合物通过激活 AIF 介导的细胞凋亡和 parthanatos 途径发挥其抗肿瘤特性。使用 U251 和 Ln229 细胞异种移植物的体内研究支持了 O6-甲基-dGTP 强大的肿瘤抑制能力。在 U87MG 细胞的原位移植模型中,与 TMZ 相比,O6-甲基-dGTP 表现出稍微优越的肿瘤抑制活性。总之,我们的研究首次强调了 O6-甲基-dGTP 作为治疗 GBM 的有效候选药物的潜力,为其在 GBM 治疗方案中的潜在临床应用奠定了坚实的科学基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号