当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of Cyclopentanes Consisting of Five Stereocenters via NHC-Catalyzed Cascade Reactions of Enals with Oxindole-Dienones
Organic Letters ( IF 4.9 ) Pub Date : 2023-11-17 , DOI: 10.1021/acs.orglett.3c03341 Yadi Niu 1 , Laiping Yao 1 , Hongli Zhao 1 , Xue Tang 1 , Qian Zhao 2 , Yuling Wu 1 , Bo Han 1 , Wei Huang 1 , Gu Zhan 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-11-17 , DOI: 10.1021/acs.orglett.3c03341 Yadi Niu 1 , Laiping Yao 1 , Hongli Zhao 1 , Xue Tang 1 , Qian Zhao 2 , Yuling Wu 1 , Bo Han 1 , Wei Huang 1 , Gu Zhan 1
Affiliation
|
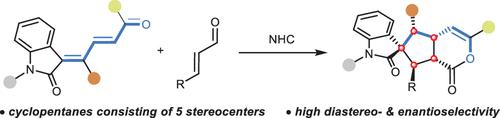
Despite the widespread presence of the chiral cyclopentane motif, the asymmetric synthesis of cyclopentanes containing five stereocenters remains a formidable challenge. Here, we present an N-heterocyclic carbene (NHC)-catalyzed cascade reaction of enal and oxindole-dienone, which allows access to spiroxindole cyclopentanes featuring a complete set of chiral centers on the five-membered carbocycle. This strategy, characterized by the formation of multiple bonds and chiral centers, demonstrates a broad substrate scope, exclusive diastereoselectivity, and up to 99:1 er.
中文翻译:

通过 NHC 催化烯醛与羟吲哚二烯酮级联反应构建由五个立体中心组成的环戊烷
尽管手性环戊烷基序广泛存在,但包含五个立构中心的环戊烷的不对称合成仍然是一个艰巨的挑战。在这里,我们提出了烯醛和羟吲哚二烯酮的N-杂环卡宾(NHC)催化级联反应,该反应可以得到在五元碳环上具有完整手性中心的螺吲哚环戊烷。该策略的特点是形成多重键和手性中心,表现出广泛的底物范围、独特的非对映选择性和高达 99:1 的选择性。
更新日期:2023-11-17
中文翻译:

通过 NHC 催化烯醛与羟吲哚二烯酮级联反应构建由五个立体中心组成的环戊烷
尽管手性环戊烷基序广泛存在,但包含五个立构中心的环戊烷的不对称合成仍然是一个艰巨的挑战。在这里,我们提出了烯醛和羟吲哚二烯酮的N-杂环卡宾(NHC)催化级联反应,该反应可以得到在五元碳环上具有完整手性中心的螺吲哚环戊烷。该策略的特点是形成多重键和手性中心,表现出广泛的底物范围、独特的非对映选择性和高达 99:1 的选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号