当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Celebration of the Publication of the 100th Volume of Organic Syntheses
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-11-17 , DOI: 10.1021/acs.jmedchem.3c01994
Margaret M Faul 1 , Kay M Brummond 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-11-17 , DOI: 10.1021/acs.jmedchem.3c01994
Margaret M Faul 1 , Kay M Brummond 2
Affiliation
|
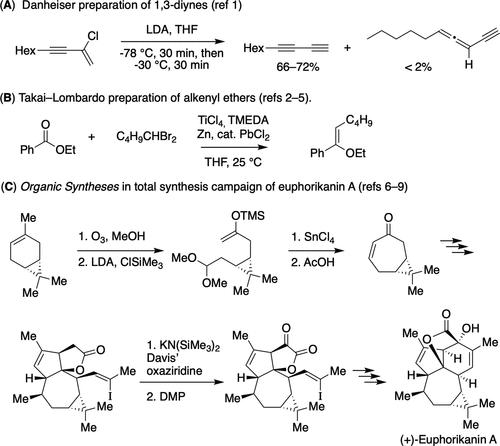
In 2023, Organic Syntheses is celebrating the publication of its 100th volume. This milestone marks a century of providing chemists with detailed, experimentally verified procedures for the synthesis of important organic compounds. The unique “validation” feature of this journal has been a valuable resource for researchers and students, promoting reproducibility and transparency in the field of organic chemistry. To celebrate this milestone, Organic Syntheses, Inc., the nonprofit publisher of the journal, hosted a symposium at the Fall ACS National Meeting in San Francisco with talks by Professor Rick Danheiser (Massachusetts Institute of Technology), Dr. Margaret Faul (Amgen, Inc.), Professor Kay Brummond (University of Pittsburgh), Professor John Wood (Baylor University), Professor Erick Carreira (ETH Zurich), Professor Pauline Chiu (University of Hong Kong), Professor Peter Wipf (University of Pittsburgh), Professor Chuck Zercher (University of New Hampshire), and Professor Scott Denmark (University of Illinois, Urbana–Champaign). This editorial will highlight the topics presented at the symposium by these contributors and describes the history, role, and future of Organic Syntheses as it continues to adapt and evolve to meet the changing needs of chemists. The first volume of Organic Syntheses was published in 1921. The procedures were collected, checked, and edited by the first Board of Editors (BOE): Roger Adams (University of Illinois), James B. Conant (Harvard University), Hans T. Clarke (Eastman Kodak Co.), and Oliver Kamm (Parke Davis). The publication was made possible through the friendship of Mr. Edward P. Hamilton of John Wiley & Sons, Inc. This was a most unusual publication venture for those times; there was no assurance that the publisher could recover the costs of the printing, binding. and distribution of this slender little “pamphlet” of 84 pages. Since that time, Organic Syntheses Inc. was established in 1939, and the resulting corporation was governed by a Board of Directors (BOD) responsible for supervising all operations of the corporation and ensuring that it complies with the Certificate of Incorporation and established bylaws. The journal has continued to provide the chemistry community with detailed, reliable, and carefully checked procedures for the synthesis of organic compounds. The role played by Organic Syntheses Inc. in promoting and improving reproducibility involves the preparation of articles in which each selected experimental procedure is checked for reproducibility in the laboratory of one of the distinguished BOE members, who are elected to serve five-to-eight-year terms. In addition to checking the procedures, the BOD and BOE also recommend articles for checking. Importantly, the Web site has an online submission process where anyone can submit a proposal for evaluation by the full BOE for practicality and suitability for future Organic Syntheses articles. On the topic of reproducibility, Professor Danheiser discussed in his ACS meeting talk that, despite unusually detailed experimental procedures, data shows that about 10% of article submissions to Organic Syntheses were not successfully checked and validated for reproducibility from 1982 to the present. He highlighted a survey by Nature in 2016 reporting that 87% of chemists tried and failed to reproduce another lab’s experiments, (1) emphasizing the value of Organic Syntheses to the chemistry community. To demonstrate the nuances learned during the development and checking of procedures for reproducibility, Danheiser cited his own lab’s work to prepare 1,3-diynes, where internal temperature control (below −70 °C) during the addition of chloroenyne to lithium diisopropylamide (LDA) was determined to be critical to a successful transformation with only trace amounts of an allene-yne side product (Scheme 1A). (2) In another example, the Takai–Lombardo alkylidenation to prepare (Z)-alkenyl ethers did not proceed in the Boeckman laboratory when using high-purity zinc available but instead required the presence of traces of lead (0.04–0.07 mol %) in the zinc powder to be effective─which led to the modern-day protocol using a catalytic amount of lead chloride (Scheme 1B). (3−5) Organic Syntheses is an important resource for the research scientist in academic laboratories developing methodology or working toward a total synthesis. Professor Carriera discussed what research scientists are looking for in synthetic methods and directly tied their needs to the value of Organic Syntheses. He made a case that research scientists want to be known for their contributions to science and for others to use their methods, a process that is reliant upon data integrity, quality, and archiving. To this end, he showcased a total synthesis campaign of euphorikanin A emanating from his laboratory (6) that relied on three experimental procedures published by others in Organic Syntheses (Scheme 1C). The seven-membered ring of the unprecedented 5/6/7/3-fused tetracyclic ring system is prepared via an ozonolysis reaction of (+)-3-carene, using an adapted Organic Syntheses protocol described by Claus and Schreiber. (7) The resulting ketone is converted to a silyl enol ether and subjected to a Lewis acid promoted intramolecular aldol condensation reaction as described by Mukaiyama and Narasaka. (8) Elaboration of the five-membered lactone to a furan-dione involves Davis’ oxaziridine reagent, (±)-trans-2-(phenylsulfonyl)-3-phenyloxazirdine. (9) With these learnings, let Organic Syntheses be one of the first places you search when you do your research! Organic Syntheses can also be used as an educational resource. Professor Chiu described how the procedures serve as a pedagogical tool in the organic chemistry ecosystem, including applications in lectures, laboratories, problem set design, and postgraduate training. She described the example of Organic Chemistry I (chem 5.12) at MIT, where experimental procedures from Organic Syntheses are presented to demonstrate reactions that are covered in class lectures, such as the syntheses of nitriles and azides via nucleophilic substitution with sodium cyanide (10) and sodium azide, (11) respectively (Scheme 2A). Another point was made that, while LDA is a convenient base to use when charting a reaction on paper, successful preparation of LDA in the lab requires specialized techniques to maintain anhydrous conditions and to safely handle pyrophoric reagents, as Professor Chiu shows in her own course using an Organic Syntheses protocol. (12) In another example from Professor Chiu, faculty at Thomas Jefferson University, Stonehill College, and Old Dominion University have used Organic Syntheses to address the lack of oxidation reactions in the introductory organic chemistry laboratory─an important functional group interconversion that has been removed from most laboratory curricula. This shortfall had been because chromium-based oxidations are traditionally taught in organic lectures but the corresponding experiments could not be included in lab courses due to the toxicity of chromium-based reagents. These chemists have therefore advocated using an oxoammonium salt (Bobbitt’s salt), written up in Organic Syntheses, (13) as the oxidant in the design of “green” and safe oxidation lab experiments (Scheme 2B). (14) Because this oxidant operates via a hydride-transfer mechanism that is opposite to that of NaBH4 and LiAlH4, a case was made for its pedagogical advantage. In another example given, Andraos has demonstrated that Organic Syntheses can be used as a reference database to calculate material efficiency metrics for linear and convergent synthetic plans─serving as an exercise that combines the teaching of green analyses and traditional learning in organic chemistry, further highlighting Organic Syntheses as a rich resource for reliable and detailed experimental procedures. (15) Advancing beyond educational resources, Professor Wipf discussed the value of Organic Syntheses in the age of data science, when >100 million terabytes of data are newly created each day by businesses, governments, and individual users of professional and entertainment devices. Organic Syntheses might be considered a relatively small database with about 7000 reactions, in comparison to SciFinder that provides data on about 150 million single- and multiple-step reactions. However, data quality is a major roadblock in mining data sets, and what makes Organic Syntheses unique in the age of data science is the quality and reliability, as well as the details of the reported data, owing to the procedures being checked and re-edited by experts. The validated procedures can be used to train machine learning models for predicting the outcome and scope of chemical reactions. This is a valuable resource for chemists who are trying to optimize their synthetic methods. An example includes prediction of chemical reaction yields based on reaction conditions using deep learning. The opportunity exists for Organic Syntheses workflows to help curate and organize data on chemical reactions by generating harmonized protocols and can be a key enabling tool for expanding quality data science in chemistry. Dr. Faul discussed the value of Organic Syntheses to the pharmaceutical industry. Since its founding in 1921, 18% of BOE members have been from industry and predominantly from process chemistry organizations. This partnership has been valuable because of the role of process chemists in developing robust reproducible reactions to support development of commercial production of medicines, and the strong emphasis on safety as one of the foundational elements of Organic Syntheses procedures. The most predominant reactions used in the pharmaceutical industry are those that are well established, Faul explained, including amide formation, SNAr reactions, Boc protection/deprotection, ester hydrolysis, and Suzuki–Miyaura coupling. (16) In medicinal chemistry, this is driven by the need to use reactions that are “tried and true” and provide an array of compounds that can be tested for efficacy and safety. In process chemistry, the drivers are to develop reactions that, beyond being reliable and safe, are cost-effective and robust in providing a high yield of product. Since Organic Syntheses has published >3000 reproducible procedures with some 7000 individual reactions, these common reaction types are covered multiple times. However, given the scope of reactions published in Organic Syntheses, there is also an opportunity for additional procedures to be applied in industrial chemistry laboratories so chemists can come to understand the breadth and scope of the reaction pool and increase the diversity of reactions for their research and high-throughput experimentation laboratories. For those involved in academic research, Faul noted that there is an opportunity to publish their reproducible procedures in Organic Syntheses, as this will support expanding the scope and complexity of compounds that can be explored across research laboratories and increase the value of the journal to the chemical community long into the future. Professor Brummond highlighted the value of the Organic Syntheses Web site to the chemistry community, as evidenced by having more than 50000 visitors per month. (17) The Web site serves as a model for transparency by providing open access, which is paid for by Organic Syntheses, Inc., to a number of resources including a rich database of experimental procedures for important synthetic transformations. (18) There is also the online submission process, where chemists are encouraged to submit their own work or to suggest the work of others, with the note that having one’s work in Organic Syntheses lowers the barrier for others to use that chemistry. (19) Articles describing the preparation of useful reagents, catalysts, and ligands or demonstrating an example of a general synthetic method or strategy are standard. But other topics such as organic material synthesis and reaction mechanism tools are welcome. The Web site is regularly updated, and Organic Syntheses Inc. continues to take steps to be more inclusive, as evidenced by the topics and authors published, the changing demographics of the BOE and BOD and the posting of a DEIR statement. (20) Several new article types are being introduced into Organic Syntheses in conjunction with the celebration of the 100th volume. In brief they include OS Perspectives─to provide authors an opportunity to provide personal perspectives on topics associated with the field of organic synthesis. OS Classics─to celebrate some of the most important chemistry published in Organic Syntheses during the past 50 years. OS Techniques─which will focus on important synthetic techniques illustrated with applications to specific organic compounds. The first one of these, “Purification of Organic Compounds by Column Chromatography”, will appear soon, so stay tuned! More than the publication of reproducible procedures, Organic Syntheses Inc. is a philanthropic organization that has invested significantly in the organic synthesis community. Professor Wood discussed how the bylaws were written to state that “the corporation is to be organized for strictly scientific, educational, and charitable purpose, and not for pecuniary profit, and no part of its net earnings will incur to the benefit of any member, director, or officer other than as reasonable compensation for services in effecting one or more such purposes.” While Organic Syntheses Inc. started with producing procedures in an annual volume for the benefit of the chemistry community, the royalties from the sale of the annual and collective volumes began to grow, and in the 1950s the philanthropic efforts of the journal were initiated. From 1955 to today, Organic Syntheses Inc. has donated $7.1 million to the organic chemistry community. The first major investment was in the Roger Adams Award in Organic Chemistry in 1956, in partnership with Organic Reactions Inc., “to recognize and encourage outstanding contributions to research in organic chemistry”. In the 1980s, as the paperback editions of the journal continued, honoraria were provided to students checking the procedures and a University Lectureship program for the BOE was initiated. In 1994, Organic Syntheses Inc. began sponsorship of the Division of Organic Chemistry Graduate Research Symposium, which continued through 2015. In the 2000s, the journal supported an endowment at Notre Dame University for the Jeremiah P. Freeman Lectureship. In 2017, Organic Syntheses Inc. initiated support for Summer Research Grants for Faculty at primarily undergraduate institutions (PUIs), and in 2019 an Organic Syntheses Inc. Workshop on Synthetic Organic Chemistry for Young Investigators started. The organization continues to support these programs in addition to making donations to several Gordon Research Conferences, the Empowering Women in Organic Chemistry conferences, and most recently endowment of the Carl R. Johnson Lectureship at Wayne State University. In fact, the synergistic combination of the original mission of Organic Syntheses Inc.─publication of checked procedures─and the journal’s philanthropic activities continue to deliver immense value to the chemistry community. There is no doubt that Organic Syntheses Inc. has had a successful 100 years. However, we need to be prepared for the future. Since the checking process has not changed significantly in 100 years, Professor Denmark addressed the question: “Is Organic Syntheses still relevant to today’s chemistry community?” With the advances in artificial intelligence, he brought us to the future and asked GPT-4 for its opinion! GPT-4 highlighted that Organic Syntheses is a respected publication that offers chemists detailed, peer-reviewed procedures for the synthesis of organic compounds. Although it has been a valuable resource for researchers and students for many years, it needs to evolve, and GPT-4 outlined several areas for consideration. Enduring relevance and the continued need for detailed and reliable procedures. Incorporate advanced techniques and technologies such as machine learning, automation, and flow chemistry. Adapt to digital editions and interactive tools to aid synthetic chemists. Provide video demonstrations to enhance accessibility and interactivity. Incorporate procedures with sustainability and green chemistry supporting environmental sustainability. GPT-4 provided excellent advice, and many of the areas highlighted have been recently discussed at the BOD and BOE meetings. As we celebrate the 100th Volume of Organic Syntheses, we are proud of the work performed by the journal and the value it has brought and can continue to bring to the organic chemistry community. Its reproducible procedures are foundational in training students, developing complex syntheses, and applying synthetic steps in the laboratories of industrial chemists. While it started with the publication of reproducible procedures for important fine chemicals, and this aspect of the journal will continue, it has grown past its need for publication revenue to fund its philanthropic activities. Advancing into the future, the BOD and BOE are well aware of the need to make changes to the journal to continue to enhance its value, and Organic Syntheses Inc. will continue to adapt and evolve to meet the changing needs of chemists, focusing on advanced methods, sustainability, and accessibility of information. The authors wish to recognize and thank all the past and current members of the Board of Directors and Board of Editors of Organic Syntheses and all the authors who have contributed their work to the journal for the benefit of the organic chemistry community. This Editorial has been copublished in The Journal of Organic Chemistry, Organic Letters, Organic Process Research & Development, Organometallics, Journal of the American Chemical Society, ACS Catalysis, Journal of Medicinal Chemistry, ACS Medicinal Chemistry Letters, and ACS Organic & Inorganic Au. This article references 20 other publications. This article has not yet been cited by other publications. This article references 20 other publications.
更新日期:2023-11-17







































 京公网安备 11010802027423号
京公网安备 11010802027423号