当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trans-cyclosulfamidate mannose-configured cyclitol allows isoform-dependent inhibition of GH47 α-D-mannosidases through a bump–hole strategy
Chemical Science ( IF 7.6 ) Pub Date : 2023-11-17 , DOI: 10.1039/d3sc05016e Alexandra Males 1 , Ken Kok 2 , Alba Nin-Hill 3, 4 , Nicky de Koster 2 , Sija van den Beukel 2 , Thomas J M Beenakker 5 , Gijsbert A van der Marel 5 , Jeroen D C Codée 5 , Johannes M F G Aerts 2 , Herman S Overkleeft 5 , Carme Rovira 3, 4 , Gideon J Davies 1 , Marta Artola 2
Chemical Science ( IF 7.6 ) Pub Date : 2023-11-17 , DOI: 10.1039/d3sc05016e Alexandra Males 1 , Ken Kok 2 , Alba Nin-Hill 3, 4 , Nicky de Koster 2 , Sija van den Beukel 2 , Thomas J M Beenakker 5 , Gijsbert A van der Marel 5 , Jeroen D C Codée 5 , Johannes M F G Aerts 2 , Herman S Overkleeft 5 , Carme Rovira 3, 4 , Gideon J Davies 1 , Marta Artola 2
Affiliation
|
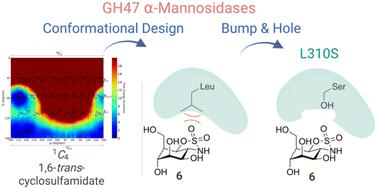
Class I inverting exo-acting α-1,2-mannosidases (CAZY family GH47) display an unusual catalytic itinerary featuring ring-flipped mannosides, 3S1 → 3H4‡ → 1C4. Conformationally locked 1C4 compounds, such as kifunensine, display nanomolar inhibition but large multigene GH47 mannosidase families render specific “isoform-dependent” inhibition impossible. Here we develop a bump-and-hole strategy in which a new mannose-configured 1,6-trans-cyclic sulfamidate inhibits α-D-mannosidases by virtue of its 1C4 conformation. This compound does not inhibit the wild-type GH47 model enzyme by virtue of a steric clash, a “bump”, in the active site. An L310S (a conserved residue amongst human GH47 enzymes) mutant of the model Caulobacter GH47 awoke 574 nM inhibition of the previously dormant inhibitor, confirmed by structural analysis of a 0.97 Å structure. Considering that L310 is a conserved residue amongst human GH47 enzymes, this work provides a unique framework for future biotechnological studies on N-glycan maturation and ER associated degradation by isoform-specific GH47 α-D-mannosidase inhibition through a bump-and-hole approach.
中文翻译:

反式环磺酰胺甘露糖配置的环醇可通过凹凸孔策略对 GH47 α-D-甘露糖苷酶进行异构体依赖性抑制
I 类反向外切作用 α-1,2-甘露糖苷酶(CAZY 家族 GH47)显示出一种不寻常的催化行程,其特点是环翻转甘露糖苷, 3 S 1 → 3 H 4 ‡ → 1 C 4 。构象锁定的1 C 4化合物(例如 kifunensine)表现出纳摩尔抑制,但大型多基因 GH47 甘露糖苷酶家族使得特定的“异构体依赖性”抑制不可能实现。在这里,我们开发了一种凹凸-孔策略,其中一种新的甘露糖配置的1,6-反式-环磺酰胺酯凭借其1 C 4构象抑制α- D-甘露糖苷酶。该化合物不会通过活性位点的空间冲突(即“碰撞”)抑制野生型 GH47 模型酶。柄杆菌GH47 模型的 L310S(人类 GH47 酶中的保守残基)突变体唤醒了先前休眠抑制剂的 574 nM 抑制,这通过 0.97 Å 结构的结构分析得到证实。考虑到 L310 是人类 GH47 酶中的保守残基,这项工作为未来生物技术研究提供了一个独特的框架,通过凹凸孔方法抑制异构体特异性 GH47 α- D-甘露糖苷酶来研究N-聚糖成熟和 ER 相关降解。
更新日期:2023-11-17
中文翻译:

反式环磺酰胺甘露糖配置的环醇可通过凹凸孔策略对 GH47 α-D-甘露糖苷酶进行异构体依赖性抑制
I 类反向外切作用 α-1,2-甘露糖苷酶(CAZY 家族 GH47)显示出一种不寻常的催化行程,其特点是环翻转甘露糖苷, 3 S 1 → 3 H 4 ‡ → 1 C 4 。构象锁定的1 C 4化合物(例如 kifunensine)表现出纳摩尔抑制,但大型多基因 GH47 甘露糖苷酶家族使得特定的“异构体依赖性”抑制不可能实现。在这里,我们开发了一种凹凸-孔策略,其中一种新的甘露糖配置的1,6-反式-环磺酰胺酯凭借其1 C 4构象抑制α- D-甘露糖苷酶。该化合物不会通过活性位点的空间冲突(即“碰撞”)抑制野生型 GH47 模型酶。柄杆菌GH47 模型的 L310S(人类 GH47 酶中的保守残基)突变体唤醒了先前休眠抑制剂的 574 nM 抑制,这通过 0.97 Å 结构的结构分析得到证实。考虑到 L310 是人类 GH47 酶中的保守残基,这项工作为未来生物技术研究提供了一个独特的框架,通过凹凸孔方法抑制异构体特异性 GH47 α- D-甘露糖苷酶来研究N-聚糖成熟和 ER 相关降解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号