当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkynyl Halo-Aza-Prins Annulative Couplings
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-11-16 , DOI: 10.1021/acs.joc.3c01305
Jackson J Hernandez 1 , Alexandra P Lawrie 1 , Alison J Frontier 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-11-16 , DOI: 10.1021/acs.joc.3c01305
Jackson J Hernandez 1 , Alexandra P Lawrie 1 , Alison J Frontier 1
Affiliation
|
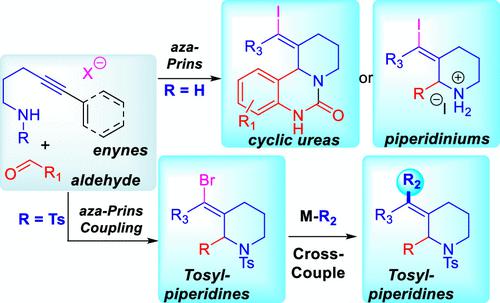
This article is a comprehensive report describing our studies in the field of aza-alkynyl Prins chemistry, comparing and contrasting the different reaction partners and reactivities observed during method development. The synthetic strategies combine an alkynyl aza-Prins coupling with an annulation, enabling the preparation of different nitrogen-containing heterocycles. Different iminium ions are explored as viable electrophiles for an alkynyl Prins cyclization, terminated by capture with a halogen nucleophile to form a vinyl halide. The synthetic utility of this functional handle is exploited through a number of Suzuki cross-couplings, allowing for the preparation of a modest library of compounds. In most cases, the Prins couplings are highly selective for the vinyl halides with E geometry, resulting from anti-addition across the alkyne.
中文翻译:

炔基卤氮杂王子环形联轴器
本文是一份综合报告,描述了我们在氮杂炔基普林斯化学领域的研究,比较和对比了方法开发过程中观察到的不同反应伙伴和反应性。该合成策略将炔基氮杂-普林斯偶联与环化相结合,从而能够制备不同的含氮杂环。不同的亚胺离子被探索作为可行的亲电子试剂用于炔基 Prins 环化,通过用卤素亲核试剂捕获来终止以形成乙烯基卤化物。该功能手柄的合成效用通过许多铃木交叉偶联得到利用,从而可以制备适度的化合物库。在大多数情况下,Prins 偶联对具有E几何结构的卤乙烯具有高度选择性,这是由于炔烃上的反加成所致。
更新日期:2023-11-16
中文翻译:

炔基卤氮杂王子环形联轴器
本文是一份综合报告,描述了我们在氮杂炔基普林斯化学领域的研究,比较和对比了方法开发过程中观察到的不同反应伙伴和反应性。该合成策略将炔基氮杂-普林斯偶联与环化相结合,从而能够制备不同的含氮杂环。不同的亚胺离子被探索作为可行的亲电子试剂用于炔基 Prins 环化,通过用卤素亲核试剂捕获来终止以形成乙烯基卤化物。该功能手柄的合成效用通过许多铃木交叉偶联得到利用,从而可以制备适度的化合物库。在大多数情况下,Prins 偶联对具有E几何结构的卤乙烯具有高度选择性,这是由于炔烃上的反加成所致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号