当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reinforcing the Electrode/Electrolyte Interphases of Lithium Metal Batteries Employing Locally Concentrated Ionic Liquid Electrolytes
Advanced Materials ( IF 27.4 ) Pub Date : 2023-11-13 , DOI: 10.1002/adma.202309062 Xu Liu 1, 2 , Alessandro Mariani 3 , Thomas Diemant 1, 2 , Maria Enrica Di Pietro 4 , Xu Dong 1, 2 , Andrea Mele 4 , Stefano Passerini 1, 2, 5
Advanced Materials ( IF 27.4 ) Pub Date : 2023-11-13 , DOI: 10.1002/adma.202309062 Xu Liu 1, 2 , Alessandro Mariani 3 , Thomas Diemant 1, 2 , Maria Enrica Di Pietro 4 , Xu Dong 1, 2 , Andrea Mele 4 , Stefano Passerini 1, 2, 5
Affiliation
|
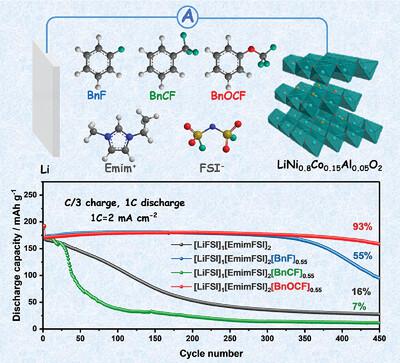
Lithium metal batteries (LMBs) with nickel-rich cathodes are promising candidates for next-generation high-energy-density batteries, but the lack of sufficiently protective electrode/electrolyte interphases (EEIs) limits their cyclability. Herein, trifluoromethoxybenzene is proposed as a cosolvent for locally concentrated ionic liquid electrolytes (LCILEs) to reinforce the EEIs. With a comparative study of a neat ionic liquid electrolyte (ILE) and three LCILEs employing fluorobenzene, trifluoromethylbenzene, or trifluoromethoxybenzene as cosolvents, it is revealed that the fluorinated groups tethered to the benzene ring of the cosolvents not only affect the electrolytes’ ionic conductivity and fluidity, but also the EEIs’ composition via adjusting the contribution of the 1-ethyl-3-methylimidazolium cation (Emim+) and bis(fluorosulfonyl)imide anion. Trifluoromethoxybenzene, as the optimal cosolvent, leads to a stable cycling of LMBs employing 5 mAh cm−2 lithium metal anodes (LMAs), 21 mg cm−2 LiNi0.8Co0.15Al0.05 (NCA) cathodes, and 4.2 µL mAh−1 electrolytes for 150 cycles with a remarkable capacity retention of 71%, thanks to a solid electrolyte interphase rich in inorganic species on LMAs and, particularly, a uniform cathode/electrolyte interphase rich in Emim+-derived species on NCA cathodes. By contrast, the capacity retention under the same condition is only 16%, 46%, and 18% for the neat ILE and the LCILEs based on fluorobenzene and benzotrifluoride, respectively.
中文翻译:

采用局部浓缩离子液体电解质增强锂金属电池的电极/电解质界面
具有富镍阴极的锂金属电池(LMB)是下一代高能量密度电池的有希望的候选者,但缺乏足够的保护性电极/电解质界面(EEI)限制了它们的循环能力。在此,三氟甲氧基苯被提议作为局部浓缩离子液体电解质(LCILE)的共溶剂以增强EEI。通过对纯离子液体电解质(ILE)和使用氟苯、三氟甲基苯或三氟甲氧基苯作为共溶剂的三种LCILE的比较研究,发现共溶剂苯环上的氟化基团不仅影响电解质的离子电导率,而且影响电解质的离子电导率。流动性,而且还通过调整 1-乙基-3-甲基咪唑鎓阳离子 (Emim + ) 和双(氟磺酰基)亚胺阴离子的贡献来调整 EEI 的组成。三氟甲氧基苯作为最佳共溶剂,可实现采用5 mAh cm -2锂金属阳极(LMA)、21 mg cm -2 LiNi 0.8 Co 0.15 Al 0.05 (NCA) 阴极和4.2 µL mAh -1电解质的LMB的稳定循环。 150 次循环后,容量保持率为 71%,这要归功于 LMA 上富含无机物质的固体电解质界面,特别是 NCA 阴极上富含 Emim +衍生物质的均匀阴极/电解质界面。相比之下,在相同条件下,纯 ILE 和基于氟苯和三氟化苯的 LCILE 的容量保留率分别仅为 16%、46% 和 18%。
更新日期:2023-11-13
中文翻译:

采用局部浓缩离子液体电解质增强锂金属电池的电极/电解质界面
具有富镍阴极的锂金属电池(LMB)是下一代高能量密度电池的有希望的候选者,但缺乏足够的保护性电极/电解质界面(EEI)限制了它们的循环能力。在此,三氟甲氧基苯被提议作为局部浓缩离子液体电解质(LCILE)的共溶剂以增强EEI。通过对纯离子液体电解质(ILE)和使用氟苯、三氟甲基苯或三氟甲氧基苯作为共溶剂的三种LCILE的比较研究,发现共溶剂苯环上的氟化基团不仅影响电解质的离子电导率,而且影响电解质的离子电导率。流动性,而且还通过调整 1-乙基-3-甲基咪唑鎓阳离子 (Emim + ) 和双(氟磺酰基)亚胺阴离子的贡献来调整 EEI 的组成。三氟甲氧基苯作为最佳共溶剂,可实现采用5 mAh cm -2锂金属阳极(LMA)、21 mg cm -2 LiNi 0.8 Co 0.15 Al 0.05 (NCA) 阴极和4.2 µL mAh -1电解质的LMB的稳定循环。 150 次循环后,容量保持率为 71%,这要归功于 LMA 上富含无机物质的固体电解质界面,特别是 NCA 阴极上富含 Emim +衍生物质的均匀阴极/电解质界面。相比之下,在相同条件下,纯 ILE 和基于氟苯和三氟化苯的 LCILE 的容量保留率分别仅为 16%、46% 和 18%。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号