当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Do Ionic Liquids Slow Down in Stages?
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-14 , DOI: 10.1021/jacs.3c08639
Bichitra Borah 1 , Gobin Raj Acharya 2 , Diana Grajeda 2 , Matthew S Emerson 1 , Matthew A Harris 3 , A M Milinda Abeykoon 4 , Joshua Sangoro 3, 5 , Gary A Baker 6 , Andrew J Nieuwkoop 2 , Claudio J Margulis 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-14 , DOI: 10.1021/jacs.3c08639
Bichitra Borah 1 , Gobin Raj Acharya 2 , Diana Grajeda 2 , Matthew S Emerson 1 , Matthew A Harris 3 , A M Milinda Abeykoon 4 , Joshua Sangoro 3, 5 , Gary A Baker 6 , Andrew J Nieuwkoop 2 , Claudio J Margulis 1
Affiliation
|
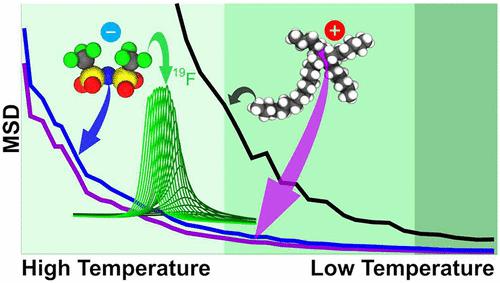
High impact recent articles have reported on the existence of a liquid–liquid (L–L) phase transition as a function of both pressure and temperature in ionic liquids (ILs) containing the popular trihexyltetradecylphosphonium cation (P666,14+), sometimes referred to as the “universal liquifier”. The work presented here reports on the structural-dynamic pathway from liquid to glass of the most well-studied IL comprising the P666,14+ cation. We present experimental and computational evidence that, on cooling, the path from the room-temperature liquid to the glass state is one of separate structural-dynamic changes. The first stage involves the slowdown of the charge network, while the apolar subcomponent is fully mobile. A second, separate stage entails the slowdown of the apolar domain. Whereas it is possible that these processes may be related to the liquid–liquid and glass transitions, more research is needed to establish this conclusively.
中文翻译:

离子液体会分阶段减速吗?
最近的高影响力文章报道了含有流行的三己基十四烷基鏻阳离子 (P 666,14 + ) 的离子液体 (IL) 中存在作为压力和温度函数的液-液 (L-L) 相变,有时也称为被誉为“万能液化剂”。这里介绍的工作报告了研究最深入的包含 P 666,14 +阳离子的 IL 从液体到玻璃的结构动力学途径。我们提供的实验和计算证据表明,在冷却时,从室温液体到玻璃态的路径是单独的结构动态变化之一。第一阶段涉及电荷网络的减慢,同时非极性子组件完全移动。第二个单独的阶段需要非极性域的减速。尽管这些过程可能与液-液和玻璃化转变有关,但需要更多的研究来确定这一点。
更新日期:2023-11-14
中文翻译:

离子液体会分阶段减速吗?
最近的高影响力文章报道了含有流行的三己基十四烷基鏻阳离子 (P 666,14 + ) 的离子液体 (IL) 中存在作为压力和温度函数的液-液 (L-L) 相变,有时也称为被誉为“万能液化剂”。这里介绍的工作报告了研究最深入的包含 P 666,14 +阳离子的 IL 从液体到玻璃的结构动力学途径。我们提供的实验和计算证据表明,在冷却时,从室温液体到玻璃态的路径是单独的结构动态变化之一。第一阶段涉及电荷网络的减慢,同时非极性子组件完全移动。第二个单独的阶段需要非极性域的减速。尽管这些过程可能与液-液和玻璃化转变有关,但需要更多的研究来确定这一点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号