当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Nucleophilic Addition to Arynes by a New Quaternary Guanidinium Salt-Based Phase-Transfer Catalyst
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-14 , DOI: 10.1021/jacs.3c09594 Guihua Pan 1 , Maoping Pu 1 , Hongyu Wang 1 , Meijia Ying 1 , Yi Li 1 , Shunxi Dong 1 , Xiaoming Feng 1 , Xiaohua Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-14 , DOI: 10.1021/jacs.3c09594 Guihua Pan 1 , Maoping Pu 1 , Hongyu Wang 1 , Meijia Ying 1 , Yi Li 1 , Shunxi Dong 1 , Xiaoming Feng 1 , Xiaohua Liu 1
Affiliation
|
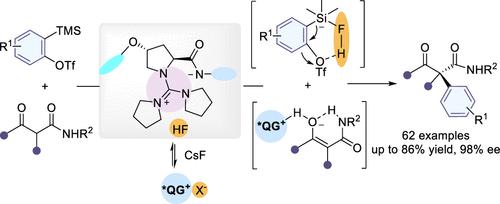
Owing to the mild generation methods, arynes have been widely used in synthetic chemistry. However, achieving asymmetric organocatalytic reactions with arynes remains a formidable and infrequent challenge, primarily because these neutral and transient species tend to spontaneously quench. To address this issue, a solid–liquid phase-transfer strategy is devised in which the generation speed of arynes could be controlled by the in situ generated fluoride-based chiral phase-transfer catalyst. In this study, we present a catalytic enantioselective nucleophilic addition reaction involving arynes, utilizing an amino amide-based guanidinium salt QG•X. Furthermore, we demonstrate the broad compatibility of this reaction with various arynes and cyclic/acyclic β-keto amides, leading to the creation of diverse α-aryl quaternary stereocenters with good stereoselectivity. Mechanistic investigations have uncovered the potential involvement of a chiral intramolecular cationic–anionic pair and HF during the ion exchange between QG•X and CsF for nucleophile activation and aryne generation. Additionally, DFT calculations suggested that the observed high levels of enantioselectivity can be attributed to steric repulsion and the cumulative noncovalent interactions between the catalysts and substrates.
中文翻译:

新型季胍盐基相转移催化剂对芳烃的催化对映选择性亲核加成
由于芳炔的生成方法温和,在合成化学中得到了广泛的应用。然而,用芳烃实现不对称有机催化反应仍然是一个艰巨且罕见的挑战,主要是因为这些中性和瞬态物质往往会自发猝灭。为了解决这个问题,设计了一种固液相转移策略,其中芳炔的生成速度可以通过原位生成的氟化物基手性相转移催化剂来控制。在这项研究中,我们利用基于氨基酰胺的胍盐QG •X,提出了一种涉及芳烃的催化对映选择性亲核加成反应。此外,我们证明了该反应与各种芳烃和环状/无环β-酮酰胺的广泛相容性,从而产生具有良好立体选择性的多种α-芳基季立体中心。机理研究发现,在QG ·X 和 CsF 之间的离子交换过程中,手性分子内阳离子-阴离子对和 HF 可能参与亲核试剂活化和芳基生成。此外,DFT 计算表明,观察到的高水平对映选择性可归因于空间排斥以及催化剂和底物之间累积的非共价相互作用。
更新日期:2023-11-14
中文翻译:

新型季胍盐基相转移催化剂对芳烃的催化对映选择性亲核加成
由于芳炔的生成方法温和,在合成化学中得到了广泛的应用。然而,用芳烃实现不对称有机催化反应仍然是一个艰巨且罕见的挑战,主要是因为这些中性和瞬态物质往往会自发猝灭。为了解决这个问题,设计了一种固液相转移策略,其中芳炔的生成速度可以通过原位生成的氟化物基手性相转移催化剂来控制。在这项研究中,我们利用基于氨基酰胺的胍盐QG •X,提出了一种涉及芳烃的催化对映选择性亲核加成反应。此外,我们证明了该反应与各种芳烃和环状/无环β-酮酰胺的广泛相容性,从而产生具有良好立体选择性的多种α-芳基季立体中心。机理研究发现,在QG ·X 和 CsF 之间的离子交换过程中,手性分子内阳离子-阴离子对和 HF 可能参与亲核试剂活化和芳基生成。此外,DFT 计算表明,观察到的高水平对映选择性可归因于空间排斥以及催化剂和底物之间累积的非共价相互作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号