当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Behavior and Data Correlation of Sulfinpyrazone in Thirteen Pure Solvents from 283.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-11-15 , DOI: 10.1021/acs.jced.3c00463 Jingxuan Qiu 1, 2 , Xinzhu Zhao 3 , Gang Liu 1, 2 , Chaoqun Zhang 1, 2 , Libo Wang 1, 2 , Yeming Wang 1, 2 , Shizhe Li 1, 2 , Lei Wang 1, 2 , Cen Yao 1, 2 , Liping Zhao 1, 2 , Peng Wang 4 , Jia Zhang 3 , Jing Lin 3 , Rui Zhang 3 , Yunchun Guo 3 , Huiliang Wang 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-11-15 , DOI: 10.1021/acs.jced.3c00463 Jingxuan Qiu 1, 2 , Xinzhu Zhao 3 , Gang Liu 1, 2 , Chaoqun Zhang 1, 2 , Libo Wang 1, 2 , Yeming Wang 1, 2 , Shizhe Li 1, 2 , Lei Wang 1, 2 , Cen Yao 1, 2 , Liping Zhao 1, 2 , Peng Wang 4 , Jia Zhang 3 , Jing Lin 3 , Rui Zhang 3 , Yunchun Guo 3 , Huiliang Wang 3
Affiliation
|
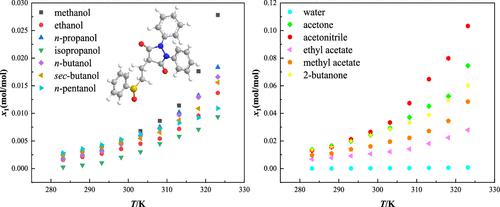
This present work is the investigation of the solubility for sulfinpyrazone in 13 monosolvents, water, methanol, ethanol, n-propanol, isopropanol, n-butanol, sec-butanol, acetone, acetonitrile, 2-butanone, methyl acetate, ethyl acetate, and n-pentanol, via a static gravimetric method at temperatures ranging from 283.15 to 323.15 K under atmospheric pressure. The solubility magnitudes are in positive correlation with the absolute temperature in each solvent. Within the experimental temperature range, the solubility is the highest in acetonitrile (103.286 × 10–3 at 323.15 K) and lowest in water (0.03597 × 10–3 at 283.15 K). The rough sequences of solubility values in different solvents are methanol > n-pentanol > n-propanol > n-butanol > sec-butanol > ethanol > isopropanol and acetonitrile > acetone > 2-butanone > methyl acetate > ethyl acetate > water in alcohols and nonalcohol solvents, respectively. The results demonstrate that the solubility behaviors of sulfinpyrazone are affected by the combined effects of several factors involving solvent polarity, solvent–solvent intermolecular interactions (quantitatively characterized by cohesive energy density), and molecular structures (molecular sizes and properties of functional groups) of solvents and solutes. Additionally, the Apelblat equation and Yaws equation were employed to correlate the experimental solubility data of sulfinpyrazone in each investigated solvent. The results of average relative deviations and root-mean-square deviations as well as the values of Akaike Information Criterion and Akaike weights reveal that the calculated solubility values by the Apelblat equation are more consistent with the experimental data compared to those by the Yaws equation.
中文翻译:

283.15 至 323.15 K 磺吡酮在 13 种纯溶剂中的溶解度行为和数据相关性
目前的工作是研究磺吡酮在 13 种单溶剂中的溶解度:水、甲醇、乙醇、正丙醇、异丙醇、正丁醇、仲丁醇、丙酮、乙腈、2-丁酮、乙酸甲酯、乙酸乙酯和正戊醇,在大气压下,在 283.15 至 323.15 K 的温度范围内通过静态重量分析法。溶解度大小与每种溶剂中的绝对温度呈正相关。在实验温度范围内,在乙腈中溶解度最高(323.15 K 时为 103.286 × 10 –3),在水中溶解度最低(283.15 K 时为 0.03597 × 10 –3)。在不同溶剂中溶解度值的粗略顺序为:甲醇>正戊醇>正丙醇>正丁醇>仲丁醇>乙醇>异丙醇和乙腈>丙酮>2-丁酮>乙酸甲酯>乙酸乙酯>醇中的水和分别为非醇溶剂。结果表明,磺吡酮的溶解行为受到溶剂极性、溶剂-溶剂分子间相互作用(通过内聚能密度定量表征)和溶剂分子结构(分子大小和官能团性质)等多种因素的综合影响。和溶质。此外,采用 Apelblat 方程和 Yaws 方程将磺吡酮在每种研究溶剂中的实验溶解度数据关联起来。平均相对偏差和均方根偏差以及Akaike信息准则和Akaike权重值的结果表明,与Yaws方程相比,Apelblat方程计算的溶解度值与实验数据更加一致。
更新日期:2023-11-15
中文翻译:

283.15 至 323.15 K 磺吡酮在 13 种纯溶剂中的溶解度行为和数据相关性
目前的工作是研究磺吡酮在 13 种单溶剂中的溶解度:水、甲醇、乙醇、正丙醇、异丙醇、正丁醇、仲丁醇、丙酮、乙腈、2-丁酮、乙酸甲酯、乙酸乙酯和正戊醇,在大气压下,在 283.15 至 323.15 K 的温度范围内通过静态重量分析法。溶解度大小与每种溶剂中的绝对温度呈正相关。在实验温度范围内,在乙腈中溶解度最高(323.15 K 时为 103.286 × 10 –3),在水中溶解度最低(283.15 K 时为 0.03597 × 10 –3)。在不同溶剂中溶解度值的粗略顺序为:甲醇>正戊醇>正丙醇>正丁醇>仲丁醇>乙醇>异丙醇和乙腈>丙酮>2-丁酮>乙酸甲酯>乙酸乙酯>醇中的水和分别为非醇溶剂。结果表明,磺吡酮的溶解行为受到溶剂极性、溶剂-溶剂分子间相互作用(通过内聚能密度定量表征)和溶剂分子结构(分子大小和官能团性质)等多种因素的综合影响。和溶质。此外,采用 Apelblat 方程和 Yaws 方程将磺吡酮在每种研究溶剂中的实验溶解度数据关联起来。平均相对偏差和均方根偏差以及Akaike信息准则和Akaike权重值的结果表明,与Yaws方程相比,Apelblat方程计算的溶解度值与实验数据更加一致。































 京公网安备 11010802027423号
京公网安备 11010802027423号