当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ionic Liquid Assisted Exothermic Complexation of Trivalent Lanthanides with Fluorinated β Diketone: Multitechnique Approach with Theoretical Insight
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-11-16 , DOI: 10.1021/acs.inorgchem.3c03029 Adityamani Nagar 1 , Arijit Sengupta 2, 3 , Musharaf Ali Sk 3, 4 , Prasanta K Mohapatra 2, 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-11-16 , DOI: 10.1021/acs.inorgchem.3c03029 Adityamani Nagar 1 , Arijit Sengupta 2, 3 , Musharaf Ali Sk 3, 4 , Prasanta K Mohapatra 2, 3
Affiliation
|
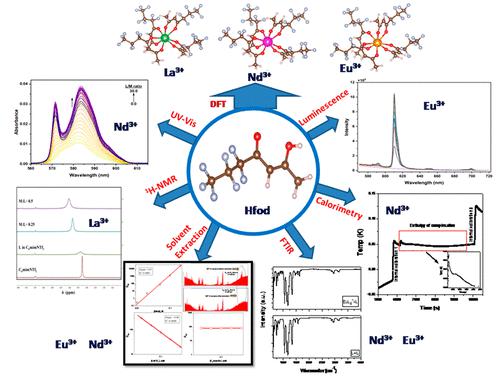
The complexation of the betadiketone,1,1,1,2,2,3,3-heptafluoro-7,7-dimethyl-4,6-octanedione (HFOD) was studied with trivalent lanthanide ions, viz. Nd3+, La3+, and Eu3+ in several methylimidazolium-based ionic liquids (Cnmim•NTf2, where, n = 4,6,8). In C6mim•NTf2, predominant formation of ML2+ and ML4– species was evidenced from the UV–vis absorption (Nd3+) as well as luminescence (Eu3+) spectral studies with log β2 ≈ 5.88 ± 0.04, log β4 ≈ 10.95 ± 0.06. The formation constants followed the trend C4mim•NTf2 > C6mim•NTf2 > C8mim•NTf2. The asymmetry factors for the ML2+ and ML4– species were found to be 1.2 and 1.59, respectively. The ML4– complex was found to have one primary coordination sphere water molecule with enhanced covalency between Eu3+ and O from HFOD (Judd Offelt constants Ω2 and Ω4 ≈ 17.2 and 2.35) compared to Eu3+aq, yet comparable to other β diketones. Complexation-induced temperature increase was confirmed by calorimetric measurements, indicating the exothermic complexation reaction (ΔHcomplexation ≈ −13.7 kJ mol–1), which is also spontaneous in nature (ΔG ≈ −68.1 kJ mol–1), with an enhancement in the entropy values. Due to complexation, the shifts in the peak positions (1686.66 cm–1, 1633.53 cm–1) associated with β diketone/ketone functional groups were evidenced. Density functional theory (DFT) calculation was performed to optimize the structural parameters including bond distance, bond angles, and energetics associated with the complexation.
中文翻译:

离子液体辅助三价镧系元素与氟化β二酮的放热络合:具有理论洞察力的多技术方法
研究了 β二酮,1,1,1,2,2,3,3-七氟-7,7-二甲基-4,6-辛二酮 (HFOD) 与三价镧系离子的络合,即。几种甲基咪唑基离子液体中的Nd 3+、La 3+和Eu 3+ (C n mim•NTf 2,其中n = 4,6,8)。在C 6 mim•NTf 2中,ML 2 +和ML 4 –物质的主要形成通过紫外可见吸收(Nd 3+)以及发光(Eu 3+)光谱研究得到证实,log β 2 ≈ 5.88 ± 0.04,log β 4 ≈ 10.95 ± 0.06。形成常数的趋势为C 4 mim•NTf 2 > C 6 mim•NTf 2 > C 8 mim•NTf 2。ML 2 +和 ML 4 –物种的不对称因子分别为 1.2 和 1.59。与 Eu 3+ aq 相比, ML 4 –配合物被发现具有一个一级配位层水分子,并且来自 HFOD 的 Eu 3+和 O 之间的共价性增强(Judd Offelt 常数 Ω 2和 Ω 4 ≈ 17.2 和 2.35),但与 Eu 3+ aq相比,仍可与其他β二酮。通过量热测量证实了络合引起的温度升高,表明放热络合反应(Δ H络合≈ -13.7 kJ mol –1),这也是自发的(Δ G ≈ -68.1 kJ mol –1),并且增强在熵值中。由于络合作用,与β二酮/酮官能团相关的峰位置(1686.66 cm –1、1633.53 cm –1 )发生了移动。进行密度泛函理论(DFT)计算以优化结构参数,包括键距、键角和与络合相关的能量学。
更新日期:2023-11-16
中文翻译:

离子液体辅助三价镧系元素与氟化β二酮的放热络合:具有理论洞察力的多技术方法
研究了 β二酮,1,1,1,2,2,3,3-七氟-7,7-二甲基-4,6-辛二酮 (HFOD) 与三价镧系离子的络合,即。几种甲基咪唑基离子液体中的Nd 3+、La 3+和Eu 3+ (C n mim•NTf 2,其中n = 4,6,8)。在C 6 mim•NTf 2中,ML 2 +和ML 4 –物质的主要形成通过紫外可见吸收(Nd 3+)以及发光(Eu 3+)光谱研究得到证实,log β 2 ≈ 5.88 ± 0.04,log β 4 ≈ 10.95 ± 0.06。形成常数的趋势为C 4 mim•NTf 2 > C 6 mim•NTf 2 > C 8 mim•NTf 2。ML 2 +和 ML 4 –物种的不对称因子分别为 1.2 和 1.59。与 Eu 3+ aq 相比, ML 4 –配合物被发现具有一个一级配位层水分子,并且来自 HFOD 的 Eu 3+和 O 之间的共价性增强(Judd Offelt 常数 Ω 2和 Ω 4 ≈ 17.2 和 2.35),但与 Eu 3+ aq相比,仍可与其他β二酮。通过量热测量证实了络合引起的温度升高,表明放热络合反应(Δ H络合≈ -13.7 kJ mol –1),这也是自发的(Δ G ≈ -68.1 kJ mol –1),并且增强在熵值中。由于络合作用,与β二酮/酮官能团相关的峰位置(1686.66 cm –1、1633.53 cm –1 )发生了移动。进行密度泛函理论(DFT)计算以优化结构参数,包括键距、键角和与络合相关的能量学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号