Developmental Cell ( IF 10.7 ) Pub Date : 2023-11-13 , DOI: 10.1016/j.devcel.2023.10.010
Yu Liu 1 , Peter John 2 , Kenta Nishitani 1 , Jihong Cui 1 , Christopher D Nishimura 2 , John R Christin 1 , Nicole Couturier 2 , Xiaoxin Ren 2 , Yao Wei 2 , Marc C Pulanco 2 , Phillip M Galbo 3 , Xusheng Zhang 4 , Wenyan Fu 1 , Wei Cui 2 , Boris A Bartholdy 5 , Deyou Zheng 6 , Gregoire Lauvau 2 , Susan A Fineberg 7 , Maja H Oktay 8 , Xingxing Zang 9 , Wenjun Guo 10
|
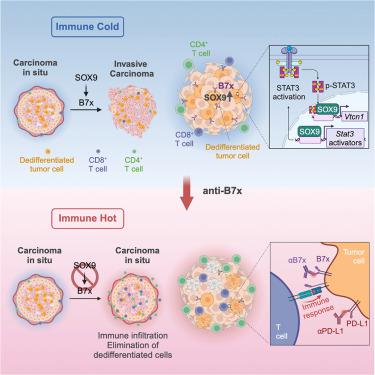
How dedifferentiated stem-like tumor cells evade immunosurveillance remains poorly understood. We show that the lineage-plasticity regulator SOX9, which is upregulated in dedifferentiated tumor cells, limits the number of infiltrating T lymphocytes in premalignant lesions of mouse basal-like breast cancer. SOX9-mediated immunosuppression is required for the progression of in situ tumors to invasive carcinoma. SOX9 induces the expression of immune checkpoint B7x/B7-H4 through STAT3 activation and direct transcriptional regulation. B7x is upregulated in dedifferentiated tumor cells and protects them from immunosurveillance. B7x also protects mammary gland regeneration in immunocompetent mice. In advanced tumors, B7x targeting inhibits tumor growth and overcomes resistance to anti-PD-L1 immunotherapy. In human breast cancer, SOX9 and B7x expression are correlated and associated with reduced CD8+ T cell infiltration. This study, using mouse models, cell lines, and patient samples, identifies a dedifferentiation-associated immunosuppression mechanism and demonstrates the therapeutic potential of targeting the SOX9-B7x pathway in basal-like breast cancer.
中文翻译:

SOX9-B7x 轴保护去分化的肿瘤细胞免受免疫监视,以驱动乳腺癌进展
去分化的干细胞样肿瘤细胞如何逃避免疫监视仍然知之甚少。我们表明,在去分化肿瘤细胞中上调的谱系可塑性调节因子 SOX9 限制了小鼠基底样乳腺癌癌前病变中浸润 T 淋巴细胞的数量。SOX9 介导的免疫抑制是原位肿瘤进展为浸润性癌所必需的。SOX9 通过 STAT3 激活和直接转录调控诱导免疫检查点 B7x/B7-H4 的表达。B7x 在去分化的肿瘤细胞中上调并保护它们免受免疫监视。B7x 还可以保护免疫功能正常小鼠的乳腺再生。在晚期肿瘤中,B7x 靶向抑制肿瘤生长并克服对抗 PD-L1 免疫疗法的耐药性。在人乳腺癌中,SOX9 和 B7x 表达与 CD8+ T 细胞浸润减少相关。这项研究使用小鼠模型、细胞系和患者样本,确定了一种与去分化相关的免疫抑制机制,并证明了在基底样乳腺癌中靶向 SOX9-B7x 通路的治疗潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号