European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-11-15 , DOI: 10.1016/j.ejmech.2023.115939 Sheng Tang 1 , Chuanchuan Sun 1 , Xintao He 1 , Wenhui Gan 1 , Linxiao Wang 1 , Dan Qiao 1 , Xinyu Guan 1 , Shan Xu 1 , Pengwu Zheng 1 , Wufu Zhu 1
|
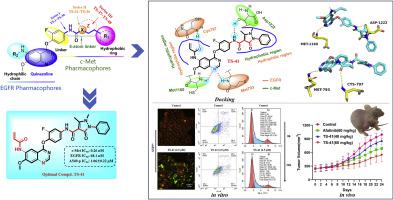
In non-small cell lung cancer (NSCLC) treatment, aberrant expression of c-mesenchymal-epithelial transition factor (c-Met) has been identified as a driving factor in epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) resistance. Unfortunately, none of the EGFR/c-Met dual-target inhibitors have successfully passed clinical trials. Hence, based on molecular docking analysis and combination principles of EGFR and c-Met inhibitors, three series of 4-(2-fluorophenoxy)-7-methoxyquinazoline derivatives as new EGFR/c-Met inhibitors were designed, synthesized, and evaluated for their biological activities. Among these compounds, TS-41 displayed the best inhibitory activity against EGFRL858R and c-Met kinases, with an IC50 value of 68.1 nM and 0.26 nM respectively. Moreover, it also showed excellent inhibitory activity on three NSCLC cell lines A549−P, H1975 and PC-9 with IC50 values ranging from 1.48 to 2.76 μM. Flow cytometry assays demonstrated that TS-41 induced apoptosis and cell cycle arrest of A549−P cells in a concentration-dependent manner, corresponding to JC-1 staining assay results. Western blot analysis revealed that TS-41 significantly downregulated the phosphorylation of EGFR, c-Met, and downstream AKT at molecular level. Importantly, TS-41 exhibited potent in vivo anticancer efficacy in an A549−P-bearing allograft nude mouse model at a dose of 60 mg/kg with a tumor growth inhibition rate of 55.3 % compared with Afatinib (46.4 %), as well as low hemolytic toxicity and organ toxicity. Molecular docking results showed that TS-41 was well embedded into the cavity of EGFR (PDB: 5GMP) and c-Met (PDB: 3LQ8) proteins, respectively. In summary, TS-41 is a high-efficiency and low-toxicity EGFR/c-Met inhibitor for the treatment of NSCLC and is worthy of further exploration.
中文翻译:

4-(2-氟苯氧基)-7-甲氧基喹唑啉衍生物作为EGFR/c-Met双重抑制剂治疗NSCLC的设计、合成和生物学评价
在非小细胞肺癌(NSCLC)治疗中,c-间充质-上皮转化因子(c-Met)的异常表达已被确定为表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKI)耐药的驱动因素。遗憾的是,目前尚未有EGFR/c-Met双靶点抑制剂成功通过临床试验。因此,基于分子对接分析以及EGFR和c-Met抑制剂的联合原理,设计、合成了三个系列的4-(2-氟苯氧基)-7-甲氧基喹唑啉衍生物作为新型EGFR/c-Met抑制剂,并评价了它们的疗效。生物活性。在这些化合物中, TS-41对EGFR L858R和c-Met激酶表现出最好的抑制活性,IC 50值分别为68.1 nM和0.26 nM。此外,它还对三种NSCLC细胞系A549 -P 、H1975和PC-9表现出优异的抑制活性,IC 50值范围为1.48至2.76 μM。流式细胞术测定表明, TS-41以浓度依赖性方式诱导 A549 -P细胞凋亡和细胞周期停滞,与 JC-1 染色测定结果相对应。 Western blot 分析显示, TS-41在分子水平上显着下调 EGFR、c-Met 和下游 AKT 的磷酸化。重要的是, TS-41在60mg/kg剂量的A549- P同种异体移植裸鼠模型中表现出有效的体内抗癌功效,与阿法替尼(46.4%)相比,肿瘤生长抑制率为55.3%,并且溶血毒性和器官毒性低。 分子对接结果显示, TS-41分别很好地嵌入到EGFR(PDB: 5GMP )和c-Met(PDB: 3LQ8 )蛋白的空腔中。综上所述, TS-41是一种高效、低毒的EGFR/c-Met抑制剂,用于治疗NSCLC,值得进一步探索。

































 京公网安备 11010802027423号
京公网安备 11010802027423号