Chem ( IF 19.1 ) Pub Date : 2023-11-14 , DOI: 10.1016/j.chempr.2023.10.008 Raquel Sánchez-Bento , Baptiste Roure , Josep Llaveria , Alessandro Ruffoni , Daniele Leonori
|
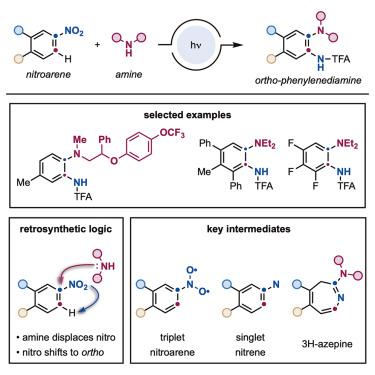
Ortho-phenylenediamines are aromatic molecules featuring two vicinal N-substituents with strong structural relevance to the development of bioactive materials. These derivatives are currently prepared from ortho-halogenated nitrobenzenes via multistep synthetic sequences. Here, we report a conceptually different approach where nitrobenzenes and amines can be directly converted into ortho-phenylenediamines without the need for ortho-halogenation and following stepwise synthetic manipulation. This strategy occurs under simple blue light irradiation and introduces an alternative retrosynthetic tactic whereby the amine coupling partner “seems” to displace the nitro group that shifts to its ortho position while being reduced and amidated in a one-pot process. Mechanistically, this process capitalizes on the conversion of nitrobenzenes into the corresponding single nitrenes, which undergo a series of N-insertion, electrocyclic ring expansion, amine addition, and electrocyclic ring contraction en route to the ortho-phenylenediamines.
中文翻译:

通过硝基苯和胺的脱芳-重芳偶联合成邻苯二胺的策略
邻位-苯二胺是具有两个邻位N-取代基的芳香族分子,与生物活性材料的开发。这些衍生物目前是由邻-卤代硝基苯通过多步合成序列制备的。在这里,我们报告了一种概念上不同的方法,其中硝基苯和胺可以直接转化为邻-苯二胺,而不需要邻位-卤化并进行逐步合成操作。该策略在简单的蓝光照射下发生,并引入了另一种逆合成策略,其中胺偶联伴侣“似乎”取代了转变为邻位的硝基邻位位置,同时在一锅法中被还原和酰胺化。从机理上讲,该过程利用硝基苯转化为相应的单一氮烯,并经历一系列N-插入、电环扩环、胺加成、和电环收缩在形成邻位-苯二胺的过程中。






























 京公网安备 11010802027423号
京公网安备 11010802027423号