Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
零电荷电势的溶剂依赖性的起源
JACS Au ( IF 8.5 ) Pub Date : 2023-11-15 , DOI: 10.1021/jacsau.3c00552
Weiqiang Tang 1, 2 , Shuangliang Zhao 1, 3 , Jun Huang 2, 4
Affiliation
|
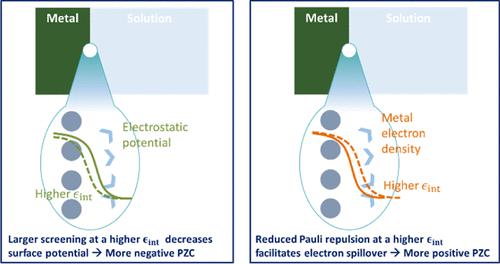
Au(111)-KPF 6界面的基本性质,特别是零电荷电势 (PZC),在溶剂之间表现出明显的变化,但其起源在很大程度上仍然难以捉摸。在这项研究中,我们的目标是将溶剂依赖性与异质介电介质中金属-溶液界面处发生的电子溢出的微观现象联系起来。为了解决在恒定电势条件下描述溶剂调制电子溢出的挑战,我们采用半经典函数方法并通过第一原理计算和实验数据对其进行参数化。我们发现,控制这种现象的关键变量是金属边缘上方约 2.5 Å 区域内的局部介电常数。较高的局部介电常数一方面有利于电子溢出,从而往往会增加 PZC,另一方面会增强对电子电荷的屏蔽,从而往往会降低 PZC。这些双重效应导致 PZC 和局部介电常数之间存在非单调关系。此外,我们的研究结果表明,电子溢出会在电极电位处产生电容峰值,该电容峰值比浓溶液中的 PZC 更负。这一观察结果与预测峰值精确出现在 PZC 的经典模型形成鲜明对比。为了阐明电子溢出对总电容的贡献,我们将总电容分解为金属的量子电容C q 、电解质溶液的经典电容C c和考虑电子-离子相关性的电容C qc 。 我们的计算表明,由于在更负的电势下促进了电子溢出, C qc为负。我们的工作不仅揭示了局部介电常数在调节电子溢出方面的重要性,而且还提出了一种可行的理论方法来研究操作条件下溶剂对电化学界面的影响。

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号