Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Versatile Platform to Generate Prodrugs with Rapid and Precise Albumin Hitchhiking and High Cargo Loading for Tumor-Targeted Chemotherapy
Small ( IF 13.0 ) Pub Date : 2023-11-14 , DOI: 10.1002/smll.202304253
Jing Li 1, 2, 3 , Huimin Xing 1 , Jie Chen 1 , Hongyu Lu 1 , Ze Tao 1, 2, 3 , Yiran Tao 4 , Yunqing Sun 1 , Tao Su 3 , Xin Li 3 , Huansheng Chang 1 , Shiyuan Chen 1 , Zhuo Chen 1 , Hao Yang 1, 2, 3 , Jingqiu Cheng 1, 2, 3 , Hong Zhu 5 , Xiaofeng Lu 1, 2, 3
Small ( IF 13.0 ) Pub Date : 2023-11-14 , DOI: 10.1002/smll.202304253
Jing Li 1, 2, 3 , Huimin Xing 1 , Jie Chen 1 , Hongyu Lu 1 , Ze Tao 1, 2, 3 , Yiran Tao 4 , Yunqing Sun 1 , Tao Su 3 , Xin Li 3 , Huansheng Chang 1 , Shiyuan Chen 1 , Zhuo Chen 1 , Hao Yang 1, 2, 3 , Jingqiu Cheng 1, 2, 3 , Hong Zhu 5 , Xiaofeng Lu 1, 2, 3
Affiliation
|
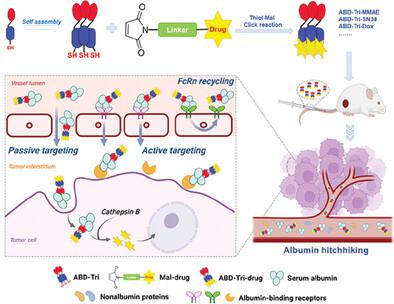
Due to its tumor homing and long serum half-life, albumin is an ideal drug carrier for chemotherapy. For endogenous albumin hitchhiking with high cargo loading, a trimeric albumin-binding domain (ABD), i.e., ABD-Tri is designed by fusing an ABD with high specificity and affinity for albumin to a self-trimerizing domain (Tri) with an additional cysteine residue. ABD-Tri is highly (40 mg L−1) expressed as soluble and trimeric proteins in Escherichia coli (E. coli). Once mixed together, ABD-Tri rapidly and specifically forms a stable complex with albumin under physiological conditions without obviously changing its receptor- and cell-binding and tumor-homing properties. Maleimide-modified prodrugs are highly effectively conjugated to ABD-Tri to produce homogenous ABD-Tri-prodrugs with triple cargo loading under physiological conditions by thiol–maleimide click chemistry. Unlike the maleimide moiety, which can only mediate time- and concentration-dependent albumin binding, ABD-Tri mediated fast (within several minutes) albumin binding of drugs even at extremely low concentrations (µg mL−1). Compared to maleimide-modified prodrugs, ABD-Tri-prodrugs exhibit better tumor homing and greater in vivo antitumor effect, indicating that conjugation of chemical drug to ABD-Tri outperforms maleimide modification for endogenous albumin hitchhiking. The results demonstrate that ABD-Tri may serve as a novel platform to produce albumin-binding prodrugs with high cargo-loading capacity for tumor-targeted chemotherapy.
中文翻译:

一个多功能平台,可通过快速、精确的白蛋白搭便车和高货物装载来生成前药,用于肿瘤靶向化疗
由于其肿瘤归巢性和较长的血清半衰期,白蛋白是化疗的理想药物载体。对于具有高负载负载的内源性白蛋白搭便车,通过将对白蛋白具有高特异性和亲和力的ABD融合到具有附加半胱氨酸的自三聚化结构域(Tri),设计了三聚体白蛋白结合结构域(ABD),即ABD-Tri残留物。 ABD-Tri 在大肠杆菌( E. coli ) 中以可溶性三聚体蛋白质的形式高度表达 (40 mg L -1 )。一旦混合在一起,ABD-Tri 在生理条件下快速、特异地与白蛋白形成稳定的复合物,而不会明显改变其受体和细胞结合以及肿瘤归巢特性。马来酰亚胺修饰的前药与 ABD-Tri 高效缀合,通过硫醇-马来酰亚胺点击化学在生理条件下产生具有三重货物负载的均质 ABD-Tri-前药。与只能介导时间和浓度依赖性白蛋白结合的马来酰亚胺部分不同,ABD-Tri 即使在极低浓度(μg mL -1 )下也能介导药物的快速(几分钟内)白蛋白结合。与马来酰亚胺修饰的前药相比,ABD-Tri-前药表现出更好的肿瘤归巢性和更强的体内抗肿瘤作用,表明化学药物与ABD-Tri的缀合在内源性白蛋白搭便车方面优于马来酰亚胺修饰。结果表明,ABD-Tri 可以作为生产具有高货物装载能力的白蛋白结合前药的新平台,用于肿瘤靶向化疗。
更新日期:2023-11-14
中文翻译:

一个多功能平台,可通过快速、精确的白蛋白搭便车和高货物装载来生成前药,用于肿瘤靶向化疗
由于其肿瘤归巢性和较长的血清半衰期,白蛋白是化疗的理想药物载体。对于具有高负载负载的内源性白蛋白搭便车,通过将对白蛋白具有高特异性和亲和力的ABD融合到具有附加半胱氨酸的自三聚化结构域(Tri),设计了三聚体白蛋白结合结构域(ABD),即ABD-Tri残留物。 ABD-Tri 在大肠杆菌( E. coli ) 中以可溶性三聚体蛋白质的形式高度表达 (40 mg L -1 )。一旦混合在一起,ABD-Tri 在生理条件下快速、特异地与白蛋白形成稳定的复合物,而不会明显改变其受体和细胞结合以及肿瘤归巢特性。马来酰亚胺修饰的前药与 ABD-Tri 高效缀合,通过硫醇-马来酰亚胺点击化学在生理条件下产生具有三重货物负载的均质 ABD-Tri-前药。与只能介导时间和浓度依赖性白蛋白结合的马来酰亚胺部分不同,ABD-Tri 即使在极低浓度(μg mL -1 )下也能介导药物的快速(几分钟内)白蛋白结合。与马来酰亚胺修饰的前药相比,ABD-Tri-前药表现出更好的肿瘤归巢性和更强的体内抗肿瘤作用,表明化学药物与ABD-Tri的缀合在内源性白蛋白搭便车方面优于马来酰亚胺修饰。结果表明,ABD-Tri 可以作为生产具有高货物装载能力的白蛋白结合前药的新平台,用于肿瘤靶向化疗。































 京公网安备 11010802027423号
京公网安备 11010802027423号