当前位置:
X-MOL 学术
›
Cancer Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A phase 2 and pharmacological study of sapanisertib in patients with relapsed and/or refractory acute lymphoblastic leukemia
Cancer Medicine ( IF 2.9 ) Pub Date : 2023-11-13 , DOI: 10.1002/cam4.6701
Aref Al-Kali 1 , Ibrahim Aldoss 2 , Pamela J Atherton 1 , Carrie A Strand 3 , Bijal Shah 4 , Jonathan Webster 5 , Bhavana Bhatnagar 6 , Karen S Flatten 7 , Kevin L Peterson 7 , Paula A Schneider 7 , Sarah A Buhrow 7 , Jianping Kong 7 , Joel M Reid 7 , Alex A Adjei 8 , Scott H Kaufmann 1, 7
Cancer Medicine ( IF 2.9 ) Pub Date : 2023-11-13 , DOI: 10.1002/cam4.6701
Aref Al-Kali 1 , Ibrahim Aldoss 2 , Pamela J Atherton 1 , Carrie A Strand 3 , Bijal Shah 4 , Jonathan Webster 5 , Bhavana Bhatnagar 6 , Karen S Flatten 7 , Kevin L Peterson 7 , Paula A Schneider 7 , Sarah A Buhrow 7 , Jianping Kong 7 , Joel M Reid 7 , Alex A Adjei 8 , Scott H Kaufmann 1, 7
Affiliation
|
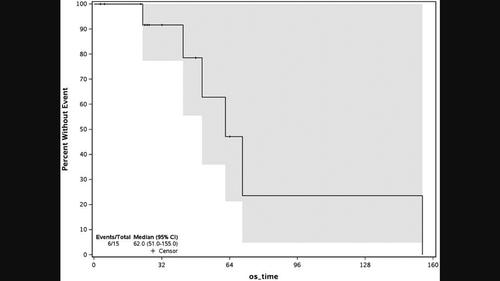
Despite recent approval of several new agents, relapsed acute lymphoblastic leukemia (ALL) remains challenging to treat. Sapanisertib (MLN0128/TAK-228) is an oral TORC1/2 inhibitor that exhibited preclinical activity against ALL.
中文翻译:

sapanisertib 在复发和/或难治性急性淋巴细胞白血病患者中的 2 期和药理研究
尽管最近批准了几种新药,但复发性急性淋巴细胞白血病 (ALL) 仍然难以治疗。Sapanisertib (MLN0128/TAK-228) 是一种口服 TORC1/2 抑制剂,对 ALL 表现出临床前活性。
更新日期:2023-11-13
中文翻译:

sapanisertib 在复发和/或难治性急性淋巴细胞白血病患者中的 2 期和药理研究
尽管最近批准了几种新药,但复发性急性淋巴细胞白血病 (ALL) 仍然难以治疗。Sapanisertib (MLN0128/TAK-228) 是一种口服 TORC1/2 抑制剂,对 ALL 表现出临床前活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号