Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 3.9 ) Pub Date : 2023-11-14 , DOI: 10.1016/j.bbalip.2023.159429
Venkatesan Ramya 1 , Karuppiah Prakash Shyam 2 , Arulanandu Angelmary 1 , Balamuthu Kadalmani 1
|
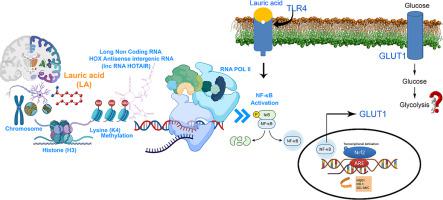
Lauric acid (LA) induces apoptosis in cancer and promotes the proliferation of normal cells by maintaining cellular redox homeostasis. Earlier, we postulated LA-mediated regulation of the NF-κB pathway by an epigenetic mechanism. However, the molecular mechanism and possible epigenetic events remained enigmatic. Herein, taking the lead from the alteration in cellular energetics in cancer cells upon LA exposure, we investigated whether LA exposure can epigenetically influence lncRNA HOTAIR, regulate glucose metabolism, and shift the cellular energetic state. Our results demonstrate LA induced modulation of lncRNA HOTAIR in a dose and time dependent manner. In addition, HOTAIR induces the expression of glucose transporter isoform 1 (GLUT1) and is regulated via NF-κB activation. Silencing HOTAIR by siRNA-mediated knockdown suppressed GLUT1 expression suggesting the key role of HOTAIR in LA-mediated metabolic reprogramming. Further, from our ChIP experiments, we observed that silencing HOTAIR subdues the recruitment of NF-κB on the GLUT1 (SLC2A1) promoter region. In addition, by performing western blot and immunocytochemistry studies, we found a dose dependent increase in Histone 3 Lysine 4 tri-methylation (H3K4me3) in the chromatin landscape. Taken together, our study demonstrates the epigenetic regulation in LA-treated SH-SY5Y cancer cells orchestrated by remodeling chromatin H3K4me3 and modulation of lncRNA HOTAIR that apparently governs the GLUT1 expression and regulates glucose uptake by exerting transcriptional control on NF-κB activation. Our work provides insights into the epigenetic regulation and metabolic reprogramming of LA through modulation of lncRNA HOTAIR, remodeling chromatin H3K4 tri-methylation, and shifting the energy metabolism in SH-SY5Y neuroblastoma cells.
中文翻译:

月桂酸通过重塑染色质 H3K4 三甲基化来表观遗传调节 lncRNA HOTAIR,并调节 SH-SY5Y 人神经母细胞瘤细胞中的葡萄糖转运:巨噬细胞激活中的脂质开关
月桂酸(LA)可诱导癌症细胞凋亡,并通过维持细胞氧化还原稳态来促进正常细胞的增殖。早些时候,我们假设 LA 介导的 NF-κB 通路通过表观遗传机制进行调节。然而,分子机制和可能的表观遗传事件仍然是个谜。在此,我们从 LA 暴露后癌细胞细胞能量学的变化出发,研究了 LA 暴露是否可以表观遗传影响 lncRNA HOTAIR、调节葡萄糖代谢并改变细胞能量状态。我们的结果表明 LA 以剂量和时间依赖性方式诱导 lncRNA HOTAIR 的调节。此外,HOTAIR 还可诱导葡萄糖转运蛋白亚型 1 (GLUT1) 的表达,并通过NF-κB 激活进行调节。通过 siRNA 介导的敲低沉默 HOTAIR 可抑制 GLUT1 表达,表明 HOTAIR 在 LA 介导的代谢重编程中的关键作用。此外,从我们的 ChIP 实验中,我们观察到沉默 HOTAIR 会抑制 GLUT1 ( SLC2A1 ) 启动子区域上 NF-κB 的募集。此外,通过进行蛋白质印迹和免疫细胞化学研究,我们发现染色质景观中组蛋白 3 赖氨酸 4 三甲基化 (H3K4me3) 呈剂量依赖性增加。总而言之,我们的研究证明了 LA 处理的 SH-SY5Y 癌细胞中的表观遗传调控是通过重塑染色质 H3K4me3 和调节 lncRNA HOTAIR 来协调的,这显然控制着 GLUT1 表达,并通过对 NF-κB 激活进行转录控制来调节葡萄糖摄取。 我们的工作通过调节 lncRNA HOTAIR、重塑染色质 H3K4 三甲基化以及改变 SH-SY5Y 神经母细胞瘤细胞的能量代谢,为 LA 的表观遗传调控和代谢重编程提供了见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号