Powder Metallurgy and Metal Ceramics ( IF 0.9 ) Pub Date : 2023-11-14 , DOI: 10.1007/s11106-023-00376-3 A. O. Synytsia , V. S. Zenkov , O. E. Sych , O. I. Khomenko , T. E. Babutina
|
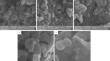
A comparative study of the morphology and physicochemical properties of magnetite synthesized by chemical precipitation for 5 min, 30 min, and 1 h and by thermolysis in nitrogen and hydrocarbon atmospheres was conducted. Regardless of the synthesis method, duration, and atmosphere, the powders were found to have spherical particles, uniform particle size distribution, and ability to agglomerate. The chemical precipitation method produced powders within a narrower size range, specifically up to 56 nm, in contrast to the thermolysis method, characterized by a particle size of up to 84 nm. Gravimetric analysis of the kinetic laws of water vapor adsorption on the synthesized powders in an air flow with a relative humidity ranging from 60 to 100% showed that the adsorption process was most intensive in the initial stage (within 30 min). The adsorption of water vapors and the process speed were significantly influenced by the synthesis method and duration and by the thermolysis atmosphere. Magnetite produced by chemical precipitation exhibited adsorption properties more than an order of magnitude higher than those of the powders produced by thermolysis. This can be attributed not only to the specific surface area but also to the material’s greater affinity for water molecules. A hydrocarbon atmosphere for thermolysis reduced the adsorption properties of magnetite by half compared to nitrogen. This may be associated not only with the potential passivation or catalytic poisoning of the powder surface but also with the influence of the carbon component on the reduction of pore volume and the promotion of magnetite adsorption capacity for polar molecules of the gaseous water phase.
中文翻译:

化学沉淀热解法制备的磁铁矿粉体对水蒸气的吸附
对化学沉淀5 min、30 min和1 h与氮气和烃气氛中热解合成的磁铁矿的形貌和物理化学性质进行了比较研究。无论合成方法、持续时间和气氛如何,粉末都具有球形颗粒、均匀的粒度分布和团聚能力。化学沉淀法生产的粉末尺寸范围较窄,特别是可达 56 nm,而热解法的颗粒尺寸可达 84 nm。对合成粉末在相对湿度60%~100%的气流中水蒸气吸附的动力学规律进行重量分析表明,吸附过程在初始阶段(30 min内)最为强烈。水蒸气的吸附和过程速度受合成方法和持续时间以及热解气氛的显着影响。通过化学沉淀产生的磁铁矿的吸附性能比通过热解产生的粉末高一个数量级以上。这不仅归因于比表面积,还归因于材料对水分子的更大亲和力。与氮气相比,用于热解的碳氢化合物气氛使磁铁矿的吸附性能降低了一半。这可能不仅与粉末表面潜在的钝化或催化中毒有关,而且还与碳组分对气态水相极性分子的孔体积减少和磁铁矿吸附能力的提高的影响有关。















































 京公网安备 11010802027423号
京公网安备 11010802027423号