当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Paramagnetic Salts of Ca, Ba, and Pb with Difurazanopyrazine and Difurazanopyrazine-N-oxide Radical Anions
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2023-11-08 , DOI: 10.1021/acs.cgd.3c01046 Galina V. Romanenko 1 , Sergey V. Fokin 1 , Artem S. Bogomyakov 1, 2 , Vitaly A. Morozov 1 , Kirill V. Strizhenko 2 , Aleksei B. Sheremetev 2 , Mikhail P. Egorov 2 , Victor I. Ovcharenko 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2023-11-08 , DOI: 10.1021/acs.cgd.3c01046 Galina V. Romanenko 1 , Sergey V. Fokin 1 , Artem S. Bogomyakov 1, 2 , Vitaly A. Morozov 1 , Kirill V. Strizhenko 2 , Aleksei B. Sheremetev 2 , Mikhail P. Egorov 2 , Victor I. Ovcharenko 1
Affiliation
|
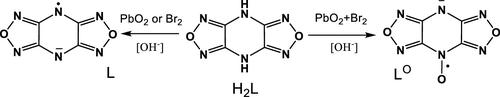
Oxidation of 4H,8H-bis(1,2,5-oxadiazolo)[3,4-b:3′,4′-e]pyrazine H2L in the presence of Ba or Ca oxide leads to the formation of paramagnetic Ba(II) and Ca(II) salts, respectively. It was shown that the basicity of the reaction mixture plays an important role in the oxidation of H2L. Molecular and crystal structures of [Ba(L)2(H2O)5]·2H2O, [Ba2(L)(HL)2(H2O)6](L)·4H2O, [Ba(HL)(H2O)6](HL)·H2O, and [Ca(L)(H2O)6]2Br2 were determined. It was found that paramagnetic [Pb(L)2(H2O)5]·2H2O can be obtained by H2L oxidation with PbO2 in the absence of alkaline earth metal oxides. It was shown that using two oxidizing agents, PbO2 and Br2, in the reaction of BaO with H2L allows us to obtain paramagnetic salts with a new anion radical bis(1,2,5-oxadiazolo)[3,4-b:3′,4′-e]pyrazine-4-oxyl (LO) and “singlet diradical” LO1. It was found that, depending on the synthetic conditions, [Ba(LO)2(H2O)6]·2H2O crystallizes in the form of several polymorphic modifications differing in the angles between the planes of LO radicals bound to the Ba ion. Quantum chemical analysis revealed that the presence of the N-oxide fragment in LO leads to a noticeable redistribution of the spin density in comparison with L. The calculated map of the distribution of exchange integrals at different relative displacements in pairs of LO allows us to give a qualitative insight into the magnetic behavior of anion radical stacks.
中文翻译:

Ca、Ba 和 Pb 与二呋喃嗪吡嗪和二呋喃嗪-N-氧化物自由基阴离子的顺磁性盐
在 Ba 或 Ca 氧化物存在下,4 H ,8 H -双(1,2,5-恶二唑)[3,4- b :3',4'- e ]吡嗪 H 2 L 的氧化导致形成分别为顺磁性 Ba(II) 盐和 Ca(II) 盐。结果表明,反应混合物的碱度对H 2 L的氧化起着重要作用。[Ba(L) 2 (H 2 O) 5 ]·2H 2 O、[Ba 2 (L)的分子和晶体结构)(HL) 2 (H 2 O) 6 ](L)·4H 2 O、[Ba(HL)(H 2 O) 6 ](HL)·H 2 O、[Ca(L)(H 2 O) ) 6 ] 2 Br 2被测定。研究发现,在不存在碱土金属氧化物的情况下,用PbO 2进行H 2 L氧化可以得到顺磁性[Pb(L) 2 (H 2 O) 5 ]·2H 2 O。结果表明,在 BaO 与 H 2 L的反应中使用两种氧化剂 PbO 2和 Br 2可以使我们获得具有新阴离子自由基双(1,2,5-恶二唑)[3,4- b :3',4'- e ]吡嗪-4-氧基 (L O ) 和“单线双自由基”L O1。研究发现,根据合成条件,[Ba(L O ) 2 (H 2 O) 6 ]·2H 2 O以几种多晶型变体的形式结晶,这些变体的L O基团平面之间的角度不同。Ba 离子。量子化学分析表明,与 L 相比, L O中N氧化物片段的存在导致自旋密度发生显着的重新分布。计算出的 L O对中不同相对位移处的交换积分分布图使我们能够定性地了解阴离子自由基堆栈的磁性行为。
更新日期:2023-11-08
中文翻译:

Ca、Ba 和 Pb 与二呋喃嗪吡嗪和二呋喃嗪-N-氧化物自由基阴离子的顺磁性盐
在 Ba 或 Ca 氧化物存在下,4 H ,8 H -双(1,2,5-恶二唑)[3,4- b :3',4'- e ]吡嗪 H 2 L 的氧化导致形成分别为顺磁性 Ba(II) 盐和 Ca(II) 盐。结果表明,反应混合物的碱度对H 2 L的氧化起着重要作用。[Ba(L) 2 (H 2 O) 5 ]·2H 2 O、[Ba 2 (L)的分子和晶体结构)(HL) 2 (H 2 O) 6 ](L)·4H 2 O、[Ba(HL)(H 2 O) 6 ](HL)·H 2 O、[Ca(L)(H 2 O) ) 6 ] 2 Br 2被测定。研究发现,在不存在碱土金属氧化物的情况下,用PbO 2进行H 2 L氧化可以得到顺磁性[Pb(L) 2 (H 2 O) 5 ]·2H 2 O。结果表明,在 BaO 与 H 2 L的反应中使用两种氧化剂 PbO 2和 Br 2可以使我们获得具有新阴离子自由基双(1,2,5-恶二唑)[3,4- b :3',4'- e ]吡嗪-4-氧基 (L O ) 和“单线双自由基”L O1。研究发现,根据合成条件,[Ba(L O ) 2 (H 2 O) 6 ]·2H 2 O以几种多晶型变体的形式结晶,这些变体的L O基团平面之间的角度不同。Ba 离子。量子化学分析表明,与 L 相比, L O中N氧化物片段的存在导致自旋密度发生显着的重新分布。计算出的 L O对中不同相对位移处的交换积分分布图使我们能够定性地了解阴离子自由基堆栈的磁性行为。








































 京公网安备 11010802027423号
京公网安备 11010802027423号