当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Benzimidazolium Salts Bearing Nitrile Moieties: Synthesis, Enzyme Inhibition Profiling, and Molecular Docking Analysis for Carbonic Anhydrase and Acetylcholinesterase
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-11-12 , DOI: 10.1002/cbdv.202301362 Erkan Öner 1 , Yetkin Gök 2, 3 , Yeliz Demir 4 , Tugba Taskin-Tok 5, 6 , Aydın Aktaş 3, 7 , İlhami Gülçin 8 , Serap Yalın 9
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-11-12 , DOI: 10.1002/cbdv.202301362 Erkan Öner 1 , Yetkin Gök 2, 3 , Yeliz Demir 4 , Tugba Taskin-Tok 5, 6 , Aydın Aktaş 3, 7 , İlhami Gülçin 8 , Serap Yalın 9
Affiliation
|
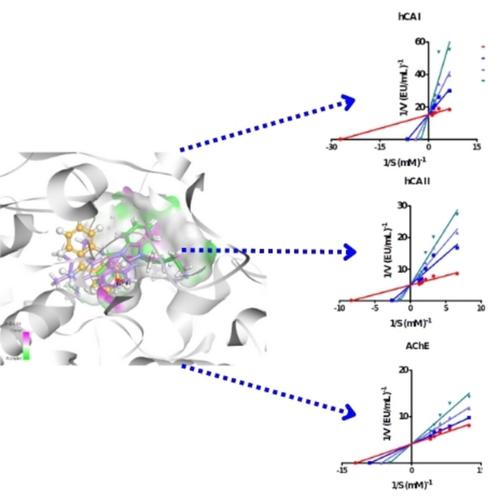
This report presents the synthesis and characterization of a range of benzimidazolium salts featuring 3-cyanopropyl groups on the 1st nitrogen atom and varied alkyl groups on the 3rd nitrogen atom within the benzimidazole structure. Benzimidazolium salts were synthesized by N-alkylation of 1-alkyl benzimidazole with 3-cyanopropyl-bromide. The new salts were characterized by 1H and 13C-NMR, FT-IR spectroscopic and elemental analysis techniques. In this study, the enzyme inhibition abilities of seven nitrile substituted benzimidazolium salts were investigated against acetylcholinesterase (AChE) and carbonic anhydrase isoenzymes I and II (hCA I and hCA II). They showed a highly potent inhibition effect on AChE, hCA I and hCA II (Ki values are in the range of 26.71–119.09 nM for AChE, 19.77 to 133.68 nM for hCA I and 13.09 to 266.38 nM for hCA II). Reflecting the binding mode of the synthesized cyanopropyl series, the importance of the 2,3,5,6-tetramethylbenzyl, 3-methylbenzyl and 3-benzyl groups for optimal interactions with target proteins, evaluated by molecular docking studies. At the same time, the docking findings support the inhibition constants (Ki) values of the related compounds in this study. Potential compounds were also evaluated by their pharmacokinetic properties were predicted.
中文翻译:

带有腈部分的苯并咪唑鎓盐:碳酸酐酶和乙酰胆碱酯酶的合成、酶抑制分析和分子对接分析
本报告介绍了一系列苯并咪唑鎓盐的合成和表征,这些苯并咪唑盐在苯并咪唑结构中的第一个氮原子上具有 3-氰基丙基,在第三个氮原子上具有不同的烷基。苯并咪唑鎓盐是通过 1-烷基苯并咪唑与 3-氰基丙基溴的 N-烷基化合成的。新盐通过1 H 和13 C-NMR、FT-IR 光谱和元素分析技术进行了表征。在本研究中,研究了七种腈取代的苯并咪唑鎓盐对乙酰胆碱酯酶(AChE)和碳酸酐酶同工酶 I 和 II(hCA I 和 hCA II)的酶抑制能力。它们对 AChE、hCA I 和 hCA II 表现出高效的抑制作用(AChE 的 K i值范围为 26.71–119.09 nM,hCA I 的 K i 值范围为 19.77 至 133.68 nM,hCA II 的 K i 值范围为 13.09 至 266.38 nM)。通过分子对接研究评估了 2,3,5,6-四甲基苯甲基、3-甲基苯甲基和 3-苯甲基对于与靶蛋白最佳相互作用的重要性,反映了合成的氰丙基系列的结合模式。同时,对接结果支持了本研究中相关化合物的抑制常数(K i )值。还通过预测潜在的化合物的药代动力学特性来评估它们。
更新日期:2023-11-12
中文翻译:

带有腈部分的苯并咪唑鎓盐:碳酸酐酶和乙酰胆碱酯酶的合成、酶抑制分析和分子对接分析
本报告介绍了一系列苯并咪唑鎓盐的合成和表征,这些苯并咪唑盐在苯并咪唑结构中的第一个氮原子上具有 3-氰基丙基,在第三个氮原子上具有不同的烷基。苯并咪唑鎓盐是通过 1-烷基苯并咪唑与 3-氰基丙基溴的 N-烷基化合成的。新盐通过1 H 和13 C-NMR、FT-IR 光谱和元素分析技术进行了表征。在本研究中,研究了七种腈取代的苯并咪唑鎓盐对乙酰胆碱酯酶(AChE)和碳酸酐酶同工酶 I 和 II(hCA I 和 hCA II)的酶抑制能力。它们对 AChE、hCA I 和 hCA II 表现出高效的抑制作用(AChE 的 K i值范围为 26.71–119.09 nM,hCA I 的 K i 值范围为 19.77 至 133.68 nM,hCA II 的 K i 值范围为 13.09 至 266.38 nM)。通过分子对接研究评估了 2,3,5,6-四甲基苯甲基、3-甲基苯甲基和 3-苯甲基对于与靶蛋白最佳相互作用的重要性,反映了合成的氰丙基系列的结合模式。同时,对接结果支持了本研究中相关化合物的抑制常数(K i )值。还通过预测潜在的化合物的药代动力学特性来评估它们。








































 京公网安备 11010802027423号
京公网安备 11010802027423号