当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In pre-clinical study fetal hypoxia caused autophagy and mitochondrial impairment in ovary granulosa cells mitigated by melatonin supplement
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2023-11-11 , DOI: 10.1016/j.jare.2023.11.008 Luyao Zhang 1 , Kexiong Liu 2 , Zhiqiang Liu 2 , Haiping Tao 3 , Xiangwei Fu 4 , Jian Hou 2 , Gongxue Jia 5 , Yunpeng Hou 2
中文翻译:

在临床前研究中,胎儿缺氧导致卵巢颗粒细胞自噬和线粒体损伤,褪黑激素补充剂减轻了这种情况
胎儿缺氧对出生后生殖功能有长期影响,卵巢颗粒细胞的线粒体损伤可能是原因之一。据报道,褪黑激素用于减轻哺乳动物细胞中的线粒体功能障碍和自噬。然而,胎儿缺氧损害新生雌性小鼠生殖功能的潜在机制以及褪黑激素对此问题的影响仍不清楚。
本研究旨在探讨胎儿缺氧损害新生雌性小鼠生殖功能的机制,并尝试通过体内和体外褪黑激素治疗来改善生殖功能。
我们建立了胎儿缺氧模型,并证实胎儿缺氧通过诱导 GC 过度自噬影响卵巢功能。进行转录组学分析、基因干扰、细胞免疫荧光、免疫组化和 western blot,以探索和验证小鼠 GCs 和 KGN 细胞的潜在机制。最后,对缺氧处理的小鼠 GCs 和 KGN 细胞进行褪黑激素处理,并向胎儿缺氧处理的小鼠注射褪黑激素以确定其效果。
体外实验结果发现,胎儿缺氧导致卵巢 GCs 线粒体功能障碍,导致自噬细胞死亡。PI3K/Akt/FoxO 通路通过对正常和胎儿缺氧小鼠卵巢 GCs 的转录组分析介导了这一过程的发生,这在小鼠 GCs 和 KGN 细胞中得到了进一步的验证。此外,褪黑激素给药可防止缺氧处理的小鼠 GC 和 KGN 细胞的自噬损伤和线粒体损伤。同时,通过褪黑激素注射进行体内实验改善了胎儿缺氧处理小鼠卵巢的氧化应激,并改善了它们的低生育能力。
我们的数据发现,胎儿缺氧导致卵巢 GCs 过度自噬,导致新生雌性小鼠生育能力低,并通过褪黑激素减轻。这些结果为缺氧应激相关的生殖障碍提供了潜在的治疗方法。
更新日期:2023-11-11
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2023-11-11 , DOI: 10.1016/j.jare.2023.11.008 Luyao Zhang 1 , Kexiong Liu 2 , Zhiqiang Liu 2 , Haiping Tao 3 , Xiangwei Fu 4 , Jian Hou 2 , Gongxue Jia 5 , Yunpeng Hou 2
Affiliation
|
Introduction
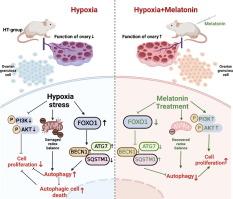
Fetal hypoxia has long-term effects on postnatal reproductive functions and the mitochondrial impairments of ovarian granulosa cells may be one of the causes. Melatonin applied to mitigate mitochondrial dysfunction and autophagy in mammalian cells has been reported. However, the potential mechanisms by which fetal hypoxia damages reproductive function in neonatal female mice and the melatonin effects on this problem remain unclear.Objectives
This research aimed to explore the mechanism that fetal hypoxia damages reproductive function in neonatal female mice and attempt to improve the reproductive function by treating with melatonin in vivo and in vitro.Methods
We established a fetal hypoxia model and confirmed that fetal hypoxia affects ovarian function by inducing GC excessive autophagy. Transcriptomic analysis, gene interference, cell immunofluorescence, immunohistochemistry and western blot were conducted to explore and verify the underlying mechanisms in mice GCs and KGN cells. Finally, melatonin treatment was executed on hypoxia-treated mice GCs and KGN cells and melatonin injection to fetal-hypoxia-treated mice to determine its effect.Results
The results of in vitro experiments found that fetal hypoxia led to mitochondrial dysfunction in ovarian GCs causing autophagic cell death. And the PI3K/Akt/FoxO pathway mediated the occurrence of this process by transcriptome analysis of ovarian GCs from normal and fetal hypoxia mice, which was further verified in mice GCs and KGN cells. Additionally, melatonin administration prevented autophagic injuries and mitochondrial impairments in hypoxia-treated mice GCs and KGN cells. Meanwhile, in vivo experiments by melatonin injection ameliorated oxidative stress of ovary in fetal-hypoxia-treated mice and improved their low fertility.Conclusion
Our data found that fetal hypoxia causes ovarian GCs excessive autophagy leading to low fertility in neonatal female mice and mitigated by melatonin. These results provide a potential therapy for hypoxic stress-related reproductive disorders.中文翻译:

在临床前研究中,胎儿缺氧导致卵巢颗粒细胞自噬和线粒体损伤,褪黑激素补充剂减轻了这种情况
介绍
胎儿缺氧对出生后生殖功能有长期影响,卵巢颗粒细胞的线粒体损伤可能是原因之一。据报道,褪黑激素用于减轻哺乳动物细胞中的线粒体功能障碍和自噬。然而,胎儿缺氧损害新生雌性小鼠生殖功能的潜在机制以及褪黑激素对此问题的影响仍不清楚。
目标
本研究旨在探讨胎儿缺氧损害新生雌性小鼠生殖功能的机制,并尝试通过体内和体外褪黑激素治疗来改善生殖功能。
方法
我们建立了胎儿缺氧模型,并证实胎儿缺氧通过诱导 GC 过度自噬影响卵巢功能。进行转录组学分析、基因干扰、细胞免疫荧光、免疫组化和 western blot,以探索和验证小鼠 GCs 和 KGN 细胞的潜在机制。最后,对缺氧处理的小鼠 GCs 和 KGN 细胞进行褪黑激素处理,并向胎儿缺氧处理的小鼠注射褪黑激素以确定其效果。
结果
体外实验结果发现,胎儿缺氧导致卵巢 GCs 线粒体功能障碍,导致自噬细胞死亡。PI3K/Akt/FoxO 通路通过对正常和胎儿缺氧小鼠卵巢 GCs 的转录组分析介导了这一过程的发生,这在小鼠 GCs 和 KGN 细胞中得到了进一步的验证。此外,褪黑激素给药可防止缺氧处理的小鼠 GC 和 KGN 细胞的自噬损伤和线粒体损伤。同时,通过褪黑激素注射进行体内实验改善了胎儿缺氧处理小鼠卵巢的氧化应激,并改善了它们的低生育能力。
结论
我们的数据发现,胎儿缺氧导致卵巢 GCs 过度自噬,导致新生雌性小鼠生育能力低,并通过褪黑激素减轻。这些结果为缺氧应激相关的生殖障碍提供了潜在的治疗方法。















































 京公网安备 11010802027423号
京公网安备 11010802027423号