Chemical Engineering Science ( IF 4.1 ) Pub Date : 2023-11-11 , DOI: 10.1016/j.ces.2023.119507
Qiang Ju , Hailing Huo , Chengxi Huang , Tongyu Wang , Xuan Liu , Zikun Liang , Liang Zhang , Jingjing Ma , Erjun Kan , Ang Li
|
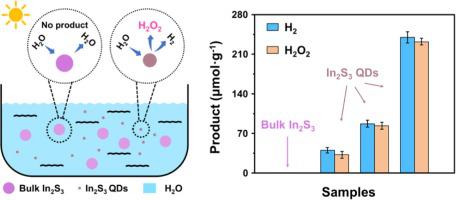
Photocatalytic overall water splitting (OWS) for H2 and H2O2 production without sacrificial agents is an appealing strategy to realize solar energy conversion. In order to realize photocatalytic OWS, it is necessary for materials to simultaneously satisfy thermodynamic and kinetic requirements, limiting the available selection scope of materials. Therefore, unlocking the potential of these materials that partially fulfill the requirements for photocatalytic overall water splitting would significantly expand the range of material choices. Here, taking In2S3 as an example, we successfully unlocked the OWS potential through a size-dependent strategy, which is based on quantum confinement effect. By adjusting its size to 2.25 nm, it can exhibit impressive photocatalytic OWS activity with the H2 and H2O2 generation rate of ∼42.17 μmol∙g−1∙h−1 and ∼38.65 μmol∙g−1∙h−1, respectively. This work provides a simple strategy to expand the range of materials selection for H2 and H2O2 production from photocatalytic OWS.
中文翻译:

解锁硫化铟的光催化整体水分解能力以产生H2和H2O2
无需牺牲剂的光催化全水分解(OWS)生产H 2和H 2 O 2是实现太阳能转换的一种有吸引力的策略。为了实现光催化OWS,材料需要同时满足热力学和动力学要求,限制了材料的选择范围。因此,释放这些部分满足光催化整体水分解要求的材料的潜力将显着扩大材料选择的范围。在这里,以 In 2 S 3为例,我们通过基于量子限制效应的尺寸依赖策略成功释放了 OWS 潜力。通过将其尺寸调整至2.25 nm,它可以表现出令人印象深刻的光催化OWS活性,H 2和H 2 O 2的生成率为∼42.17 μmol∙g −1 ∙h −1和∼38.65 μmol∙g −1 ∙h −1, 分别。这项工作提供了一个简单的策略来扩大光催化OWS生产H 2和H 2 O 2的材料选择范围。

































 京公网安备 11010802027423号
京公网安备 11010802027423号