Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2023-11-10 , DOI: 10.1016/j.bmc.2023.117530 Guiying Wu 1 , Hui Zhong 1 , Ying Wang 1 , Li Chen 1 , Jianbo Sun 1
|
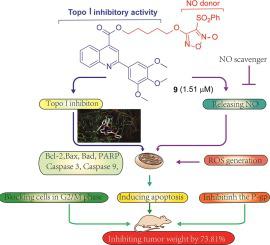
A number of NO-releasing quinoline derivatives have been designed and synthesized by introducing NO donor to quinoline carboxylic acid fragment. The anti-proliferation of all target compounds was evaluated against human cancer cell lines (HCT-116, MCF-7, and A549), MCF-7/ADR and normal cell (MCF-10A). Most compounds showed cytotoxic activity on cancer cells and drug-resistant cells with IC50 values in the range of 0.62–5.51 μM. Importantly, these compounds showed low toxicity to normal cells (4.21–34.08 μM). Further mechanism studies showed that the most potent compound 9 could release high concentration of NO and inhibit the activity of topoisomerase I. In addition, 9 regulated apoptosis-related proteins, generated ROS and blocked MCF-7 cells in G2/M phase to induce cell apoptosis. Furthermore, the P-gp-mediated transport was also influenced by 9. And 9 could significantly inhibit the growth of tumor in vivo without observable organ-related toxicities. Overall, as a novel NO-releasing quinoline derivative, 9 was worthy for further in-depth study.
中文翻译:

通过抑制拓扑异构酶 I 诱导人乳腺癌细胞凋亡的新型喹啉-NO 供体杂交体的开发
通过将NO供体引入喹啉羧酸片段,设计并合成了许多释放NO的喹啉衍生物。所有目标化合物的抗增殖作用均针对人类癌细胞系(HCT-116、MCF-7 和 A549)、MCF-7/ADR 和正常细胞(MCF-10A)进行了评估。大多数化合物对癌细胞和耐药细胞表现出细胞毒活性,IC 50值在0.62–5.51 μM 范围内。重要的是,这些化合物对正常细胞表现出低毒性(4.21–34.08 μM)。进一步的机制研究表明,最强效的化合物9可以释放高浓度的NO并抑制拓扑异构酶I的活性。此外, 9还调节凋亡相关蛋白,产生ROS并阻断MCF-7细胞处于G2/M期,诱导细胞凋亡。细胞凋亡。此外,P-gp 介导的转运也受到9 的影响。 9可显着抑制体内肿瘤的生长,且未观察到与器官相关的毒性。总体而言, 9作为一种新型的释放NO的喹啉衍生物,值得进一步深入研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号