European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-11-10 , DOI: 10.1016/j.ejmech.2023.115907 Yodita Asfaha 1 , Lukas M Bollmann 1 , Alexander J Skerhut 1 , Fabian Fischer 1 , Nadine Horstick 1 , Dennis Roth 2 , Maria Wecker 2 , Christian Mammen 3 , Sander H J Smits 4 , Georg Fluegen 2 , Matthias U Kassack 1 , Thomas Kurz 1
|
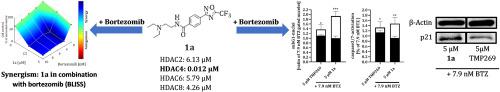
Clinically used pan and class I HDACi cause severe side effects, whereas class IIa HDACi are less cytotoxic. Here, we present the synthesis and anticancer effects of a series of 5-(trifluoromethyl)-1,2,4-oxadiazole (TFMO)-based amides and alkoxyamides derived from the previously reported class IIa HDACi YAK540. The most active class IIa inhibitor 1a showed nanomolar inhibition of the class IIa enzymes 4, 5, 7 (IC50 HDAC4: 12 nM) and high selectivity (selectivity index >318 for HDAC4) over non-class IIa HDACs. Instead of a hydroxamic acid group, 1a has a trifluoromethyloxadiazolyl (TFMO) moiety as a non-chelating Zinc-binding group (ZBG). Applying the Chou-Talalay-method we found an increased synergistic cytotoxic effect of 1a in combination with bortezomib in THP1 cells. 1a in combination with bortezomib enhanced expression of p21 leading to increased caspase-induced apoptosis. Eventually, growth inhibition by 1a of the head-neck cancer cell line Cal27 was increased upon HDAC4 overexpression in Cal27 in cell culture and using the in vivo chorioallantoic membrane model. The class IIa HDACi 1a outperforms previously described HDAC class IIa inhibitor YAK540 regarding anticancer effects and may constitute a novel option compared to pan and class I HDACi in anticancer combination treatments.
中文翻译:

基于 5-(三氟甲基)-1,2,4-恶二唑 (TFMO) 的高选择性 IIa 类 HDAC 抑制剂与硼替佐米联合显示出协同抗癌活性
临床上使用的 pan 和 I 类 HDACi会引起严重的副作用,而 IIa 类 HDACi 的细胞毒性较小。在这里,我们展示了一系列源自先前报道的 IIa 类 HDACi YAK540 的 5-(三氟甲基)-1,2,4-恶二唑 (TFMO) 基酰胺和烷氧基酰胺的合成和抗癌作用。与非 IIa 类 HDAC 相比,最具活性的 IIa 类抑制剂1a显示出对 IIa 类酶 4、5、7 的纳摩尔抑制(IC 50 HDAC4:12 nM)和高选择性(HDAC4 的选择性指数 >318)。 1a具有三氟甲基恶二唑基 (TFMO) 部分作为非螯合锌结合基团 (ZBG) ,而不是异羟肟酸基团。应用 Chou-Talalay 方法,我们发现1a与硼替佐米联合使用在 THP1 细胞中增强了协同细胞毒性作用。 1a与硼替佐米联合增强 p21 的表达,导致 caspase 诱导的细胞凋亡增加。最终,在细胞培养物中和使用体内绒毛尿囊膜模型中,Cal27 中 HDAC4 过表达后, 1a对头颈癌细胞系 Cal27 的生长抑制增加。 IIa 类 HDACi 1a在抗癌作用方面优于先前描述的 HDAC IIa 类抑制剂 YAK540,并且与泛和 I 类 HDACi 相比,在抗癌联合治疗中可能构成一种新的选择。































 京公网安备 11010802027423号
京公网安备 11010802027423号