Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Salt Anion Amphiphilicity-Activated Electrolyte Cosolvent Selection Strategy toward Durable Zn Metal Anode
ACS Nano ( IF 15.8 ) Pub Date : 2023-11-10 , DOI: 10.1021/acsnano.3c08716 Liyang Liu 1, 2, 3 , Haiying Lu 2 , Chao Han 4 , Xianfei Chen 5 , Sucheng Liu 2 , Jiakui Zhang 2 , Xianghong Chen 2 , Xinyi Wang 3 , Rui Wang 2 , Jiantie Xu 2 , Hua Kun Liu 3, 6 , Shi Xue Dou 3, 6 , Weijie Li 1, 3
ACS Nano ( IF 15.8 ) Pub Date : 2023-11-10 , DOI: 10.1021/acsnano.3c08716 Liyang Liu 1, 2, 3 , Haiying Lu 2 , Chao Han 4 , Xianfei Chen 5 , Sucheng Liu 2 , Jiakui Zhang 2 , Xianghong Chen 2 , Xinyi Wang 3 , Rui Wang 2 , Jiantie Xu 2 , Hua Kun Liu 3, 6 , Shi Xue Dou 3, 6 , Weijie Li 1, 3
Affiliation
|
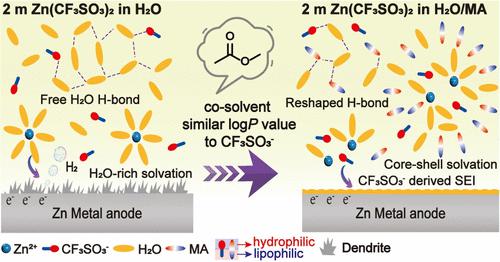
One effective solution to inhibit side reactions and Zn dendrite growth in aqueous Zn-ion batteries is to add a cosolvent into the Zn(CF3SO3)2 electrolyte, which has the potential to form a robust solid electrolyte interface composed of ZnF2 and ZnS. Nevertheless, there is still a lack of discussion on a convenient selection method for cosolvents, which can directly reflect the interactions between solvent and solute to rationally design the electrolyte solvation structure. Herein, logP, where P is the octanol–water partition coefficient, a general parameter to describe the hydrophilicity and lipophilicity of chemicals, is proposed as a standard for selecting cosolvents for Zn(CF3SO3)2 electrolyte, which is demonstrated by testing seven different types of solvents. The solvent with a logP value similar to that of the salt anion CF3SO3– can interact with CF3SO3–, Zn2+, and H2O, leading to a reconstruction of the electrolyte solvation structure. To prove the concept, methyl acetate (MA) is demonstrated as an example due to its similar logP value to that of CF3SO3–. Both the experimental and theoretical results illustrate that MA molecules not only enter into the solvation shell of CF3SO3– but also coordinate with Zn2+ or H2O, forming an MA and CF3SO3– involved core–shell solvation structure. The special solvation structure reduces H2O activity and contributes to forming an anion-induced ZnCO3–ZnF2-rich solid electrolyte interface. As a result, the Zn||Zn cell and Zn||NaV3O8·1.5H2O cell with MA-involved electrolyte exhibit superior performances to that with the MA-free electrolyte. This work provides an insight into electrolyte design via salt anion chemistry for high-performance Zn batteries.
中文翻译:

盐阴离子两亲性激活电解质共溶剂选择策略以获得耐用的锌金属阳极
抑制水系锌离子电池中副反应和锌枝晶生长的一种有效解决方案是在 Zn(CF 3 SO 3 ) 2电解质中添加共溶剂,该电解质有可能形成由 ZnF 2和ZnF 2组成的坚固的固体电解质界面。硫化锌。然而,目前仍缺乏一种方便的助溶剂选择方法,能够直接反映溶剂与溶质之间的相互作用,从而合理设计电解质溶剂化结构。这里,log P,其中P是辛醇-水分配系数,是描述化学品亲水性和亲油性的通用参数,被提议作为选择Zn(CF 3 SO 3 ) 2电解质助溶剂的标准,其证明如下:测试七种不同类型的溶剂。log P值与盐阴离子CF 3 SO 3 –相似的溶剂可以与CF 3 SO 3 –、Zn 2+和H 2 O相互作用,导致电解质溶剂化结构的重建。为了证明这一概念,以乙酸甲酯 (MA) 为例进行演示,因为它的 log P值与 CF 3 SO 3 –相似。实验和理论结果均表明MA分子不仅进入CF 3 SO 3的溶剂化壳层,而且还与Zn 2+或H 2 O配位,形成MA和CF 3 SO 3参与的核壳溶剂化结构。特殊的溶剂化结构降低了H 2 O活性,有助于形成阴离子诱导的富含ZnCO 3 -ZnF 2 的固体电解质界面。结果,含有MA电解质的Zn||Zn电池和Zn||NaV 3 O 8 ·1.5H 2 O电池表现出比不含MA电解质的电池更优异的性能。这项工作通过盐阴离子化学为高性能锌电池提供了电解质设计的见解。
更新日期:2023-11-10
中文翻译:

盐阴离子两亲性激活电解质共溶剂选择策略以获得耐用的锌金属阳极
抑制水系锌离子电池中副反应和锌枝晶生长的一种有效解决方案是在 Zn(CF 3 SO 3 ) 2电解质中添加共溶剂,该电解质有可能形成由 ZnF 2和ZnF 2组成的坚固的固体电解质界面。硫化锌。然而,目前仍缺乏一种方便的助溶剂选择方法,能够直接反映溶剂与溶质之间的相互作用,从而合理设计电解质溶剂化结构。这里,log P,其中P是辛醇-水分配系数,是描述化学品亲水性和亲油性的通用参数,被提议作为选择Zn(CF 3 SO 3 ) 2电解质助溶剂的标准,其证明如下:测试七种不同类型的溶剂。log P值与盐阴离子CF 3 SO 3 –相似的溶剂可以与CF 3 SO 3 –、Zn 2+和H 2 O相互作用,导致电解质溶剂化结构的重建。为了证明这一概念,以乙酸甲酯 (MA) 为例进行演示,因为它的 log P值与 CF 3 SO 3 –相似。实验和理论结果均表明MA分子不仅进入CF 3 SO 3的溶剂化壳层,而且还与Zn 2+或H 2 O配位,形成MA和CF 3 SO 3参与的核壳溶剂化结构。特殊的溶剂化结构降低了H 2 O活性,有助于形成阴离子诱导的富含ZnCO 3 -ZnF 2 的固体电解质界面。结果,含有MA电解质的Zn||Zn电池和Zn||NaV 3 O 8 ·1.5H 2 O电池表现出比不含MA电解质的电池更优异的性能。这项工作通过盐阴离子化学为高性能锌电池提供了电解质设计的见解。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号