当前位置:
X-MOL 学术
›
JACC Heart Fail.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tafamidis Efficacy Among Octogenarian Patients in the Phase 3 ATTR-ACT and Ongoing Long-Term Extension Study
JACC: Heart Failure ( IF 10.3 ) Pub Date : 2023-11-08 , DOI: 10.1016/j.jchf.2023.08.032 Pablo Garcia-Pavia 1 , Marla B Sultan 2 , Balarama Gundapaneni 3 , Yoshiki Sekijima 4 , Federico Perfetto 5 , Mazen Hanna 6 , Ronald Witteles 7
JACC: Heart Failure ( IF 10.3 ) Pub Date : 2023-11-08 , DOI: 10.1016/j.jchf.2023.08.032 Pablo Garcia-Pavia 1 , Marla B Sultan 2 , Balarama Gundapaneni 3 , Yoshiki Sekijima 4 , Federico Perfetto 5 , Mazen Hanna 6 , Ronald Witteles 7
Affiliation
|
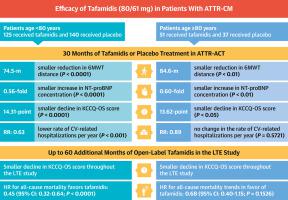
Tafamidis was approved to treat patients with transthyretin amyloid cardiomyopathy (ATTR-CM) on the basis of findings from the phase 3 Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT). This study was a post hoc analysis exploring tafamidis efficacy in octogenarian patients. Analysis of patients aged <80 and ≥80 years in ATTR-ACT and its ongoing open-label long-term extension (LTE) study, where all patients receive tafamidis. After 30 months in ATTR-ACT, least squares (LS) mean change from baseline in 6-minute walk test (6MWT) distance, N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration, and Kansas City Cardiomyopathy Questionnaire Overall Summary (KCCQ-OS) score were smaller (all 0.05) in patients aged ≥80 years treated with tafamidis (n = 51) vs placebo (n = 37). At the LTE study interim analysis, patients aged ≥80 years treated continuously with tafamidis had a smaller decline in KCCQ-OS score ( 0.05) and trended toward longer median survival (45 vs 27 months; all-cause mortality HR: 0.6828 [95% CI: 0.4048-1.1517]; 0.1526) than those initially treated with placebo in ATTR-ACT. Similar efficacy was observed in patients aged <80 years in ATTR-ACT, including smaller LS mean change from baseline in 6MWT distance, NT-proBNP concentration, and KCCQ-OS score, and lower rate of cardiovascular-related hospitalizations with tafamidis (n = 125) vs placebo (n = 140). In the LTE study, patients aged <80 years treated continuously with tafamidis had a longer median survival (80 vs 41 months; HR = 0.4513 [95% CI: 0.3176-0.6413]; 0.0001) and a smaller decline in KCCQ-OS score than those initially treated with placebo. The findings demonstrate tafamidis efficacy for patients with ATTR-CM both in those aged <80 and those aged ≥80 years. (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial [ATTR-ACT]; /Long-term Safety of Tafamidis in Subjects With Transthyretin Cardiomyopathy; )
中文翻译:

3 期 ATTR-ACT 和正在进行的长期扩展研究中 Tafamidis 在八十几岁患者中的疗效
基于转甲状腺素蛋白心肌病临床试验 (ATTR-ACT) 3 期 Tafamidis 的结果,Tafamidis 被批准用于治疗转甲状腺素蛋白淀粉样变心肌病 (ATTR-CM) 患者。这项研究是一项事后分析,探讨了他法米迪对八旬老人的疗效。 ATTR-ACT 及其正在进行的开放标签长期扩展 (LTE) 研究中对 <80 和 ≥80 岁患者的分析,其中所有患者均接受 tafamidis。 ATTR-ACT 30 个月后,6 分钟步行测试 (6MWT) 距离中最小二乘法 (LS) 相对于基线的平均变化、N 末端 B 型利钠肽原 (NT-proBNP) 浓度以及堪萨斯城心肌病问卷与安慰剂 (n = 37) 相比,年龄≥80 岁的患者接受他法米迪 (n = 51) 治疗的总体总结 (KCCQ-OS) 评分较小(均为 0.05)。在 LTE 研究中期分析中,持续接受 tafamidis 治疗的年龄≥80 岁的患者 KCCQ-OS 评分下降幅度较小(0.05),并且中位生存期趋于更长(45 个月 vs 27 个月;全因死亡率 HR:0.6828 [95%] CI:0.4048-1.1517];0.1526)比 ATTR-ACT 中最初使用安慰剂治疗的患者要高。在 ATTR-ACT 中,在年龄为 <80 岁的患者中观察到了类似的疗效,包括 6MWT 距离、NT-proBNP 浓度和 KCCQ-OS 评分相对于基线的 LS 平均变化较小,以及服用他法米迪的心血管相关住院率较低 (n = 125) 与安慰剂 (n = 140)。在 LTE 研究中,持续接受 tafamidis 治疗的 <80 岁患者的中位生存期更长(80 个月 vs 41 个月;HR = 0.4513 [95% CI: 0.3176-0.6413];0.0001),并且 KCCQ-OS 评分下降幅度较小。那些最初接受安慰剂治疗的人。研究结果表明,tafamidis 对 <80 岁和 ≥80 岁的 ATTR-CM 患者均有效。(Tafamidis 在运甲状腺素蛋白心肌病临床试验中 [ATTR-ACT];/Tafamidis 在运甲状腺素蛋白心肌病受试者中的长期安全性;)
更新日期:2023-11-08
中文翻译:

3 期 ATTR-ACT 和正在进行的长期扩展研究中 Tafamidis 在八十几岁患者中的疗效
基于转甲状腺素蛋白心肌病临床试验 (ATTR-ACT) 3 期 Tafamidis 的结果,Tafamidis 被批准用于治疗转甲状腺素蛋白淀粉样变心肌病 (ATTR-CM) 患者。这项研究是一项事后分析,探讨了他法米迪对八旬老人的疗效。 ATTR-ACT 及其正在进行的开放标签长期扩展 (LTE) 研究中对 <80 和 ≥80 岁患者的分析,其中所有患者均接受 tafamidis。 ATTR-ACT 30 个月后,6 分钟步行测试 (6MWT) 距离中最小二乘法 (LS) 相对于基线的平均变化、N 末端 B 型利钠肽原 (NT-proBNP) 浓度以及堪萨斯城心肌病问卷与安慰剂 (n = 37) 相比,年龄≥80 岁的患者接受他法米迪 (n = 51) 治疗的总体总结 (KCCQ-OS) 评分较小(均为 0.05)。在 LTE 研究中期分析中,持续接受 tafamidis 治疗的年龄≥80 岁的患者 KCCQ-OS 评分下降幅度较小(0.05),并且中位生存期趋于更长(45 个月 vs 27 个月;全因死亡率 HR:0.6828 [95%] CI:0.4048-1.1517];0.1526)比 ATTR-ACT 中最初使用安慰剂治疗的患者要高。在 ATTR-ACT 中,在年龄为 <80 岁的患者中观察到了类似的疗效,包括 6MWT 距离、NT-proBNP 浓度和 KCCQ-OS 评分相对于基线的 LS 平均变化较小,以及服用他法米迪的心血管相关住院率较低 (n = 125) 与安慰剂 (n = 140)。在 LTE 研究中,持续接受 tafamidis 治疗的 <80 岁患者的中位生存期更长(80 个月 vs 41 个月;HR = 0.4513 [95% CI: 0.3176-0.6413];0.0001),并且 KCCQ-OS 评分下降幅度较小。那些最初接受安慰剂治疗的人。研究结果表明,tafamidis 对 <80 岁和 ≥80 岁的 ATTR-CM 患者均有效。(Tafamidis 在运甲状腺素蛋白心肌病临床试验中 [ATTR-ACT];/Tafamidis 在运甲状腺素蛋白心肌病受试者中的长期安全性;)













































 京公网安备 11010802027423号
京公网安备 11010802027423号