Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2023-11-06 , DOI: 10.1016/j.bmcl.2023.129543 Yudai Imai 1 , Ryo Suzuki 1 , Daisuke Matsuda 1 , Nozomi Tanaka-Yamamoto 1 , Yuta Ohki 1 , Ryotaro Tabata 2 , Sota Kato 2 , Mami Sugisaki 2 , Natsuko Fujimoto 3 , Takuya Fukunaga 3 , Sayaka Kato 3 , Teisuke Takahashi 2 , Hiroyuki Kakinuma 1
|
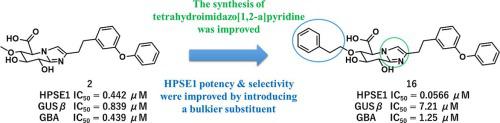
Heparanase-1 (HPSE1) is an endo-β-d-glucuronidase that catalyzes degradation of heparan sulfate proteoglycans. Inhibition of HPSE1 appears to be a useful therapeutic target against cancer and proteinuric kidney diseases. We previously reported tetrahydroimidazo[1,2-a]pyridine 2 as a potent HPSE1 inhibitor after optimization of the synthetic reaction. However, synthesis of 2 involves a total of 19 steps, including a cyclization process that accompanies a strong odor due to the use of Lawesson’s reagent and an epimerization reaction; furthermore, 2 exhibited insufficient selectivity for HPSE1 over exo-β-d-glucuronidase (GUSβ) and glucocerebrosidase (GBA), which also needed to be addressed. First, the cyclization reaction was optimized to synthesize tetrahydroimidazo[1,2-a]pyridine without using Lawesson’s reagent or epimerization, with reference to previous reports. Next, 16 and 17 containing a bulkier substituent at position 6 than the 6-methoxyl group in 2 were designed and synthesized using the improved cyclization conditions, so that the synthetic route of 16 and 17 was shortened by five steps as compared with that of 2. The inhibitory activities of 16 and 17 against GUSβ and GBA were reduced as compared with those of 2, that is, the compounds showed improved selectivity for HPSE1 over GUSβ and GBA. In addition, 16 showed enhanced inhibitory activity against HPSE1 as compared with that of 2. Compound 16 appears promising as an HPSE1 inhibitor with therapeutic potential due to its highly potent inhibitory activity against HPSE1 with high selectivity for HPSE1.
中文翻译:

利用改进的合成方法发现一种新的四氢咪唑[1,2-a]吡啶-5-羧酸衍生物作为一种有效的选择性乙酰肝素酶-1 抑制剂
乙酰肝素酶-1 (HPSE1) 是一种内切 β-d-葡萄糖醛酸酶,可催化硫酸乙酰肝素蛋白聚糖的降解。抑制 HPSE1 似乎是针对癌症和蛋白尿性肾病的有用治疗靶点。我们之前报道了四氢咪唑[1,2-a]吡啶 2 在合成反应优化后作为有效的 HPSE1 抑制剂。然而,2 的合成总共涉及 19 个步骤,包括由于使用 Lawesson 试剂和差向异构化反应而伴随着强烈气味的环化过程;此外,2 个对 HPSE1 的选择性低于外β-d-葡萄糖醛酸酶 (GUSβ) 和葡糖脑苷脂酶 (GBA),这也需要解决。首先,参考以前的报道,优化环化反应以合成四氢咪唑并[1,2-a]吡啶,而无需使用 Lawesson 试剂或差向异构化。接下来,设计并在 6 位包含比 2 中的 6-甲氧基更庞大的取代基的 16 和 17 使用改进的环化条件合成,使 16 和 17 的合成路线与 2 的合成路线相比缩短了 5 个步骤。与 2 相比,16 和 17 对 GUSβ 和 GBA 的抑制活性降低,即化合物对 HPSE1 的选择性高于 GUSβ 和 GBA。此外,与 2 相比,16 对 HPSE1 的抑制活性增强。化合物 16 作为具有治疗潜力的 HPSE1 抑制剂似乎很有希望,因为它对 HPSE1 具有高效的抑制活性,对 HPSE1 具有高选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号