当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct Electrochemical Synthesis of Acetamide from CO2 and N2 on a Single-Atom Alloy Catalyst
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-11-07 , DOI: 10.1021/acsami.3c11097 Jingnan Wang 1 , Sha Li 2 , Qiang Liu 3 , Kaiheng Zhao 1 , Yongan Yang 1 , Xi Wang 4
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-11-07 , DOI: 10.1021/acsami.3c11097 Jingnan Wang 1 , Sha Li 2 , Qiang Liu 3 , Kaiheng Zhao 1 , Yongan Yang 1 , Xi Wang 4
Affiliation
|
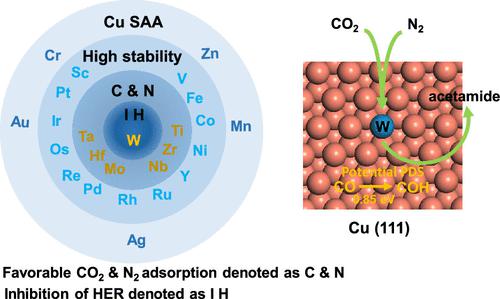
The electrochemical conversion of carbon dioxide into value-added compounds not only paves the way toward a sustainable society but also unlocks the potential for electrocatalytic synthesis of amides through the introduction of N atoms. However, it also poses one of the greatest challenges in catalysis: achieving simultaneous completion of C–C coupling and C–N coupling. Here, we have meticulously investigated the catalytic prowess of Cu-based single-atom alloys in facilitating the electrochemical synthesis of acetamide from CO2 and N2. Through a comprehensive screening process encompassing catalyst stability, adsorption capability, and selectivity against the HER, W/Cu(111) SAA has emerged as an auspicious contender. The reaction entails CO2 reduction to CO, C–C coupling leading to the formation of a ketene intermediate *CCO, N2 reduction, and C–N coupling between NH3 and *CCO culminating in the production of acetamide. The W/Cu(111) surface not only exhibits exceptional activity in the formation of acetamide, with a barrier energy of 0.85 eV for the rate-determining CO hydrogenation step, but also effectively suppresses undesired side reactions leading to various C1 and C2 byproducts during CO2 reduction. This work presents a highly effective approach for forming C–C and C–N bonds via coelectroreduction of CO2 and N2, illuminating the reaction mechanism underlying acetamide synthesis from these two gases on single-atom alloy catalysts. The catalyst design strategy employed in this study has the potential to be extended to a range of amide chemicals, thereby broadening the scope of products that can be obtained through CO2/N2 reduction.
中文翻译:

单原子合金催化剂上 CO2 和 N2 直接电化学合成乙酰胺
将二氧化碳电化学转化为增值化合物不仅为可持续社会铺平了道路,而且还通过引入氮原子释放了电催化合成酰胺的潜力。然而,它也提出了催化中最大的挑战之一:实现C-C偶联和C-N偶联的同时完成。在这里,我们仔细研究了铜基单原子合金在促进从CO 2和N 2电化学合成乙酰胺方面的催化能力。通过涵盖催化剂稳定性、吸附能力和 HER 选择性的全面筛选过程,W/Cu(111) SAA 已成为一个有利的竞争者。该反应需要CO 2还原为CO,C-C 偶联导致烯酮中间体*CCO 的形成,N 2还原以及NH 3和*CCO 之间的C-N 偶联,最终产生乙酰胺。W/Cu(111)表面不仅在乙酰胺的形成中表现出优异的活性,对于决定速率的CO氢化步骤具有0.85 eV的势垒能,而且还有效地抑制了导致各种C 1和C 2的不需要的副反应。 CO 2还原过程中的副产物。这项工作提出了一种通过CO 2和N 2共电还原形成C-C和C-N键的高效方法,阐明了在单原子合金催化剂上从这两种气体合成乙酰胺的反应机理。本研究中采用的催化剂设计策略有可能扩展到一系列酰胺化学品,从而扩大通过CO 2 /N 2还原获得的产品范围。
更新日期:2023-11-07
中文翻译:

单原子合金催化剂上 CO2 和 N2 直接电化学合成乙酰胺
将二氧化碳电化学转化为增值化合物不仅为可持续社会铺平了道路,而且还通过引入氮原子释放了电催化合成酰胺的潜力。然而,它也提出了催化中最大的挑战之一:实现C-C偶联和C-N偶联的同时完成。在这里,我们仔细研究了铜基单原子合金在促进从CO 2和N 2电化学合成乙酰胺方面的催化能力。通过涵盖催化剂稳定性、吸附能力和 HER 选择性的全面筛选过程,W/Cu(111) SAA 已成为一个有利的竞争者。该反应需要CO 2还原为CO,C-C 偶联导致烯酮中间体*CCO 的形成,N 2还原以及NH 3和*CCO 之间的C-N 偶联,最终产生乙酰胺。W/Cu(111)表面不仅在乙酰胺的形成中表现出优异的活性,对于决定速率的CO氢化步骤具有0.85 eV的势垒能,而且还有效地抑制了导致各种C 1和C 2的不需要的副反应。 CO 2还原过程中的副产物。这项工作提出了一种通过CO 2和N 2共电还原形成C-C和C-N键的高效方法,阐明了在单原子合金催化剂上从这两种气体合成乙酰胺的反应机理。本研究中采用的催化剂设计策略有可能扩展到一系列酰胺化学品,从而扩大通过CO 2 /N 2还原获得的产品范围。







































 京公网安备 11010802027423号
京公网安备 11010802027423号