当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphate and myo-Inositol Hexakisphosphate Adsorption onto Hematite as Affected by Ca2+, Mg2+, and pH
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2023-11-09 , DOI: 10.1021/acsearthspacechem.3c00192 Ruan F. Firmano 1 , J. Derek Peak 2 , Michael P. Schmidt 3 , Luís R. F. Alleoni 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2023-11-09 , DOI: 10.1021/acsearthspacechem.3c00192 Ruan F. Firmano 1 , J. Derek Peak 2 , Michael P. Schmidt 3 , Luís R. F. Alleoni 1
Affiliation
|
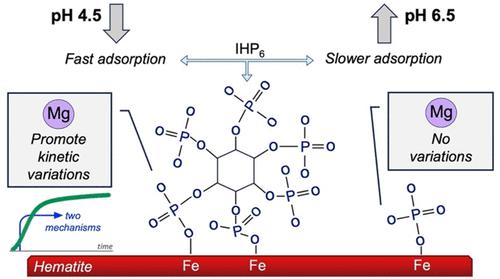
myo-Inositol hexakisphosphate (IHP6) is typically the most abundant form of organic phosphorus (Po) in soils, and this species is highly reactive with Al and Fe (hydr)oxides because of the six phosphoryl groups in its structure. In this study, the effects of pH (4.5–6.5) and cations (Ca2+ and Mg2+) were investigated using in situ attenuated total reflectance Fourier transform infrared (ATR–FTIR) spectroscopy and Mg K-edge X-ray absorption spectroscopy (XAS). IHP6 has more complex infrared (IR) spectra than inorganic phosphate, even though both share certain bands of absorbances. Ca and Mg influenced the IHP6 adsorption process on the hematite surface, but this effect was less evident for phosphate. The variation in pH promoted a shift of several bands in the adsorbed IR spectra. As for adsorption kinetics, IHP6 was sensitive to a pH change from 4.5 to 6.5, with a reduction in the adsorption rate at the highest pH. Phosphate, on the other hand, presented more rapid adsorption kinetics than IHP6, with equilibrium reached in ∼80 min. Two adsorption mechanisms were identified for IHP6 and phosphate kinetics, without the influence of the studied cations. However, differences in the shape of XAS spectra at the Mg K-edge revealed that there was a probable change in the atomic environment of Mg2+ caused by its association with phosphate. In other evaluated organic molecules, such as IHP6 and citrate, this effect was not observed as a result of the intense presence of MgCl2 in the linear combination fitting (LCF) analysis. In general, the results enhance the molecular-level understanding of oxyanion adsorption onto hematite surfaces that can predict IHP6 and phosphate behaviors in iron-oxide-rich soils.
中文翻译:

磷酸盐和肌醇六磷酸盐在赤铁矿上的吸附受 Ca2+、Mg2+ 和 pH 的影响
肌醇六磷酸 (IHP 6 ) 通常是土壤中最丰富的有机磷 (P o ) 形式,并且由于其结构中有六个磷酰基,该物质与铝和铁(氢)氧化物具有高度反应性。在本研究中,利用原位衰减全反射傅里叶变换红外 (ATR-FTIR) 光谱和 Mg K边 X 射线吸收研究了pH (4.5–6.5) 和阳离子(Ca 2+和 Mg 2+ )的影响光谱(XAS)。IHP 6具有比无机磷酸盐更复杂的红外 (IR) 光谱,尽管两者共享某些吸收带。Ca和Mg影响IHP 6在赤铁矿表面的吸附过程,但这种影响对于磷酸盐不太明显。pH 值的变化促进了吸收红外光谱中几个谱带的移动。至于吸附动力学,IHP 6对 pH 从 4.5 到 6.5 的变化很敏感,在最高 pH 下吸附速率会降低。另一方面,磷酸盐表现出比 IHP 6更快的吸附动力学,在约 80 分钟内达到平衡。确定了 IHP 6和磷酸盐动力学的两种吸附机制,不受所研究的阳离子的影响。然而,Mg K边缘的 XAS 光谱形状的差异表明,由于Mg 2+与磷酸盐的结合,其原子环境可能发生了变化。在其他评估的有机分子中,例如 IHP 6和柠檬酸盐,由于线性组合拟合 (LCF) 分析中大量存在 MgCl 2 ,因此未观察到这种效应。总的来说,这些结果增强了对赤铁矿表面氧阴离子吸附的分子水平理解,可以预测富含氧化铁的土壤中的IHP 6和磷酸盐行为。
更新日期:2023-11-09
中文翻译:

磷酸盐和肌醇六磷酸盐在赤铁矿上的吸附受 Ca2+、Mg2+ 和 pH 的影响
肌醇六磷酸 (IHP 6 ) 通常是土壤中最丰富的有机磷 (P o ) 形式,并且由于其结构中有六个磷酰基,该物质与铝和铁(氢)氧化物具有高度反应性。在本研究中,利用原位衰减全反射傅里叶变换红外 (ATR-FTIR) 光谱和 Mg K边 X 射线吸收研究了pH (4.5–6.5) 和阳离子(Ca 2+和 Mg 2+ )的影响光谱(XAS)。IHP 6具有比无机磷酸盐更复杂的红外 (IR) 光谱,尽管两者共享某些吸收带。Ca和Mg影响IHP 6在赤铁矿表面的吸附过程,但这种影响对于磷酸盐不太明显。pH 值的变化促进了吸收红外光谱中几个谱带的移动。至于吸附动力学,IHP 6对 pH 从 4.5 到 6.5 的变化很敏感,在最高 pH 下吸附速率会降低。另一方面,磷酸盐表现出比 IHP 6更快的吸附动力学,在约 80 分钟内达到平衡。确定了 IHP 6和磷酸盐动力学的两种吸附机制,不受所研究的阳离子的影响。然而,Mg K边缘的 XAS 光谱形状的差异表明,由于Mg 2+与磷酸盐的结合,其原子环境可能发生了变化。在其他评估的有机分子中,例如 IHP 6和柠檬酸盐,由于线性组合拟合 (LCF) 分析中大量存在 MgCl 2 ,因此未观察到这种效应。总的来说,这些结果增强了对赤铁矿表面氧阴离子吸附的分子水平理解,可以预测富含氧化铁的土壤中的IHP 6和磷酸盐行为。































 京公网安备 11010802027423号
京公网安备 11010802027423号