当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures and biological activity of three 2-(pyridin-2-yl)-1H-benzimidazole derivatives
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2023-11-08 , DOI: 10.1107/s2053229623009452 Jarosław Sukiennik 1 , Andrzej Olczak 1 , Katarzyna Gobis 2 , Izabela Korona-Głowniak 3 , Katarzyna Suśniak 3 , Andrzej Fruziński 1 , Małgorzata Szczesio 1
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2023-11-08 , DOI: 10.1107/s2053229623009452 Jarosław Sukiennik 1 , Andrzej Olczak 1 , Katarzyna Gobis 2 , Izabela Korona-Głowniak 3 , Katarzyna Suśniak 3 , Andrzej Fruziński 1 , Małgorzata Szczesio 1
Affiliation
|
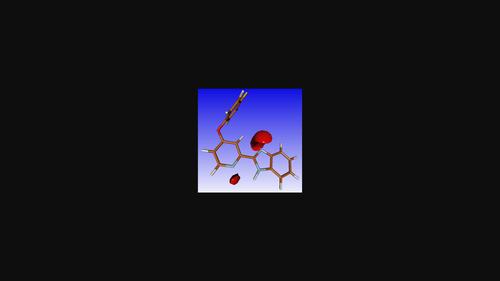
Two new 2-(pyridin-2-yl)-1H-benzimidazole derivatives, namely, 2-(4-phenoxypyridin-2-yl)-1H-benzimidazole, C18H13N3O, and 2-[4-(4-fluorophenoxy)pyridin-2-yl]-1H-benzimidazole, C18H12FN3O, were synthesized and characterized by NMR spectroscopy. Crystal structure, biological activity and ADME analyses were performed for these two new compounds and a third compound, namely, 5,6-dimethyl-2-[4-(4-phenylpiperazin-1-yl)pyridin-2-yl]-1H-benzimidazole methanol monosolvate, C24H25N5·CH3OH, the synthesis of which had been described previously. All three compounds have a similar chain hydrogen-bonding pattern. One of them (the fluorophenoxy derivative) showed good antimicrobial activity against Gram-positive bacteria. The ADME analysis indicates that the compounds could be good drug candidates.
中文翻译:

三种2-(吡啶-2-基)-1H-苯并咪唑衍生物的结构和生物活性
两种新的2-(吡啶-2-基)-1 H-苯并咪唑衍生物,即2-(4-苯氧基吡啶-2-基)-1 H-苯并咪唑、C 18 H 13 N 3 O和2-[4合成了-(4-氟苯氧基)吡啶-2-基]-1 H-苯并咪唑C 18 H 12 FN 3 O,并通过NMR光谱进行表征。对这两种新化合物和第三种化合物(即 5,6-二甲基-2-[4-(4-苯基哌嗪-1-基)吡啶-2-基]-1)进行了晶体结构、生物活性和 ADME 分析。 H-苯并咪唑甲醇单溶剂化物C 24 H 25 N 5 ·CH 3 OH,其合成先前已描述。所有三种化合物都具有相似的链氢键模式。其中一种(氟苯氧基衍生物)对革兰氏阳性菌表现出良好的抗菌活性。 ADME 分析表明这些化合物可能是良好的候选药物。
更新日期:2023-11-08
中文翻译:

三种2-(吡啶-2-基)-1H-苯并咪唑衍生物的结构和生物活性
两种新的2-(吡啶-2-基)-1 H-苯并咪唑衍生物,即2-(4-苯氧基吡啶-2-基)-1 H-苯并咪唑、C 18 H 13 N 3 O和2-[4合成了-(4-氟苯氧基)吡啶-2-基]-1 H-苯并咪唑C 18 H 12 FN 3 O,并通过NMR光谱进行表征。对这两种新化合物和第三种化合物(即 5,6-二甲基-2-[4-(4-苯基哌嗪-1-基)吡啶-2-基]-1)进行了晶体结构、生物活性和 ADME 分析。 H-苯并咪唑甲醇单溶剂化物C 24 H 25 N 5 ·CH 3 OH,其合成先前已描述。所有三种化合物都具有相似的链氢键模式。其中一种(氟苯氧基衍生物)对革兰氏阳性菌表现出良好的抗菌活性。 ADME 分析表明这些化合物可能是良好的候选药物。































 京公网安备 11010802027423号
京公网安备 11010802027423号