Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-11-08 , DOI: 10.1016/j.bioorg.2023.106952
Yiquan Wu 1 , Mingfei Wu 1 , Xiaoli Zheng 2 , Hengyuan Yu 3 , Xinfei Mao 1 , Yuyuan Jin 4 , Yanhong Wang 5 , Ao Pang 1 , Jingyu Zhang 1 , Shenxin Zeng 4 , Tengfei Xu 3 , Yong Chen 3 , Bo Zhang 6 , Nengming Lin 6 , Haibin Dai 5 , Yuwei Wang 7 , Xiaojun Yao 8 , Xiaowu Dong 9 , Wenhai Huang 4 , Jinxin Che 1
|
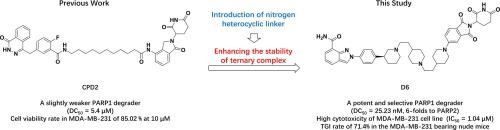
PARP1 is a multifaceted component of DNA repair and chromatin remodeling, making it an effective therapeutic target for cancer therapy. The recently reported proteolytic targeting chimera (PROTAC) could effectively degrade PARP1 through the ubiquitin–proteasome pathway, expanding the therapeutic application of PARP1 blocking. In this study, a series of nitrogen heterocyclic PROTACs were designed and synthesized through ternary complex simulation analysis based on our previous work. Our efforts have resulted in a potent PARP1 degrader D6 (DC50 = 25.23 nM) with high selectivity due to nitrogen heterocyclic linker generating multiple interactions with the PARP1-CRBN PPI surface, specifically. Moreover, D6 exhibited strong cytotoxicity to triple negative breast cancer cell line MDA-MB-231 (IC50 = 1.04 µM). And the proteomic results showed that the antitumor mechanism of D6 was found that intensifies DNA damage by intercepting the CDC25C-CDK1 axis to halt cell cycle transition in triple-negative breast cancer cells. Furthermore, in vivo study, D6 showed a promising PK property with moderate oral absorption activity. And D6 could effectively inhibit tumor growth (TGI rate = 71.4 % at 40 mg/kg) without other signs of toxicity in MDA-MB-321 tumor-bearing mice. In summary, we have identified an original scaffold and potent PARP1 PROTAC that provided a novel intervention strategy for the treatment of triple-negative breast cancer.
中文翻译:

发现一种有效的选择性 PARP1 降解剂,通过拦截 CDC25C-CDK1 轴促进细胞周期停滞,用于治疗三阴性乳腺癌
PARP1 是 DNA 修复和染色质重塑的多方面成分,使其成为癌症治疗的有效治疗靶点。最近报道的蛋白水解靶向嵌合体(PROTAC)可以通过泛素-蛋白酶体途径有效降解PARP1,扩大了PARP1阻断的治疗应用。本研究在前期工作的基础上,通过三元配合物模拟分析,设计并合成了一系列氮杂环PROTAC。我们的努力已经产生了一种有效的 PARP1 降解剂D6 (DC 50 = 25.23 nM),由于氮杂环连接基与 PARP1-CRBN PPI 表面产生多重相互作用,因此具有高选择性。此外, D6对三阴性乳腺癌细胞系 MDA-MB-231 表现出强细胞毒性 (IC 50 = 1.04 µM)。蛋白质组学结果表明, D6的抗肿瘤机制是通过拦截CDC25C-CDK1轴来加剧DNA损伤,从而阻止三阴性乳腺癌细胞的细胞周期转变。此外,在体内研究中, D6显示出良好的 PK 特性和中等的口服吸收活性。 D6可以有效抑制 MDA-MB-321 荷瘤小鼠的肿瘤生长(40 mg/kg 时,TGI 率为 71.4%),且没有其他毒性迹象。总之,我们已经确定了一种原始支架和有效的 PARP1 PROTAC,为三阴性乳腺癌的治疗提供了一种新颖的干预策略。






































 京公网安备 11010802027423号
京公网安备 11010802027423号