当前位置:
X-MOL 学术
›
ACS Nanosci. Au
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Iron Phosphide Nanobundles from an Iron Oxyhydroxide Precursor
ACS Nanoscience Au ( IF 4.8 ) Pub Date : 2023-11-08 , DOI: 10.1021/acsnanoscienceau.3c00036 Menuka Adhikari 1 , Shubham Sharma 1 , Elena Echeverria 2 , David N McIlroy 2 , Yolanda Vasquez 1
ACS Nanoscience Au ( IF 4.8 ) Pub Date : 2023-11-08 , DOI: 10.1021/acsnanoscienceau.3c00036 Menuka Adhikari 1 , Shubham Sharma 1 , Elena Echeverria 2 , David N McIlroy 2 , Yolanda Vasquez 1
Affiliation
|
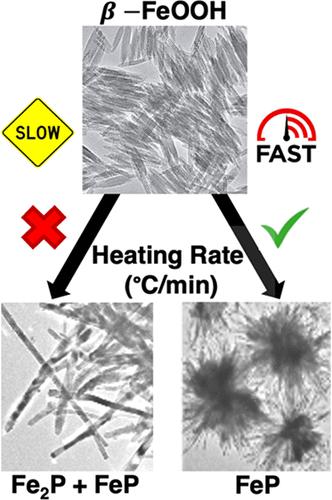
Iron phosphide (FeP) nanoparticles have excellent properties such as fast charge transfer kinetics, high electrical conductivity, and high stability, making them a promising catalyst for hydrogen evolution reaction (HER). A challenge to the wide use of iron phosphide nanomaterials for this application is the available synthesis protocols that limit control over the resulting crystalline phase of the product. In this study, we report a method for synthesizing FeP through a solution-based process. Here, we use iron oxyhydroxide (β-FeOOH) as a cost-effective, environmentally friendly, and air-stable source of iron, along with tri-n-octylphosphine (TOP) as the phosphorus source and solvent. FeP is formed in a nanobundle morphology in the solution phase reaction at a temperature of 320 °C. The materials were characterized by pXRD and transmission electron microscopy (TEM). The optimization parameters evaluated to produce the phosphorus-rich FeP phase included the reaction rate, time, amount of TOP, and reaction temperature. Mixtures of Fe2P and FeP phases were obtained at shorter reaction times and slow heating rates (4.5 °C /min), while longer reaction times and faster heating rates (18.8 °C/min) favored the formation of phosphorus-rich FeP. Overall, the reaction lever that consistently yielded FeP as the predominant crystalline phase was a fast heat rate.
中文翻译:

由羟基氧化铁前体形成磷化铁纳米束
磷化铁(FeP)纳米粒子具有快速电荷转移动力学、高导电性和高稳定性等优异性能,使其成为析氢反应(HER)的有前途的催化剂。磷化铁纳米材料在该应用中广泛使用的一个挑战是可用的合成方案限制了对所得产品晶相的控制。在这项研究中,我们报告了一种通过基于溶液的过程合成 FeP 的方法。在这里,我们使用羟基氧化铁(β-FeOOH)作为一种经济高效、环境友好且空气稳定的铁源,并使用三正辛基膦(TOP)作为磷源和溶剂。 FeP 在 320 °C 温度下的溶液相反应中以纳米束形态形成。通过 pXRD 和透射电子显微镜 (TEM) 对材料进行了表征。评估生产富磷 FeP 相的优化参数包括反应速率、时间、TOP 量和反应温度。 Fe 2 P和FeP相的混合物在较短的反应时间和较慢的加热速率(4.5℃/min)下获得,而较长的反应时间和较快的加热速率(18.8℃/min)有利于富磷FeP的形成。总体而言,持续产生 FeP 作为主要晶相的反应杠杆是快速加热速率。
更新日期:2023-11-08
中文翻译:

由羟基氧化铁前体形成磷化铁纳米束
磷化铁(FeP)纳米粒子具有快速电荷转移动力学、高导电性和高稳定性等优异性能,使其成为析氢反应(HER)的有前途的催化剂。磷化铁纳米材料在该应用中广泛使用的一个挑战是可用的合成方案限制了对所得产品晶相的控制。在这项研究中,我们报告了一种通过基于溶液的过程合成 FeP 的方法。在这里,我们使用羟基氧化铁(β-FeOOH)作为一种经济高效、环境友好且空气稳定的铁源,并使用三正辛基膦(TOP)作为磷源和溶剂。 FeP 在 320 °C 温度下的溶液相反应中以纳米束形态形成。通过 pXRD 和透射电子显微镜 (TEM) 对材料进行了表征。评估生产富磷 FeP 相的优化参数包括反应速率、时间、TOP 量和反应温度。 Fe 2 P和FeP相的混合物在较短的反应时间和较慢的加热速率(4.5℃/min)下获得,而较长的反应时间和较快的加热速率(18.8℃/min)有利于富磷FeP的形成。总体而言,持续产生 FeP 作为主要晶相的反应杠杆是快速加热速率。































 京公网安备 11010802027423号
京公网安备 11010802027423号