当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery, Structure, and Mechanism of the (R, S)-Norcoclaurine Synthase for the Chiral Synthesis of Benzylisoquinoline Alkaloids
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-11-08 , DOI: 10.1021/acscatal.3c03296 Libo Zhang 1 , Shiqing Zhang 2, 3 , Lijing Liao 4 , Huanying Tang 1 , Wenli Wang 5 , Fucheng Yin 6 , Liangliang Han 6 , Kejin Zhu 1 , Yushi Liu 1 , Dingqiao Xu 7 , Xiaobing Wang 6 , Minjian Qin 1 , Yibei Xiao 4 , Xiang Sheng 2, 3 , Yucheng Zhao 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-11-08 , DOI: 10.1021/acscatal.3c03296 Libo Zhang 1 , Shiqing Zhang 2, 3 , Lijing Liao 4 , Huanying Tang 1 , Wenli Wang 5 , Fucheng Yin 6 , Liangliang Han 6 , Kejin Zhu 1 , Yushi Liu 1 , Dingqiao Xu 7 , Xiaobing Wang 6 , Minjian Qin 1 , Yibei Xiao 4 , Xiang Sheng 2, 3 , Yucheng Zhao 1
Affiliation
|
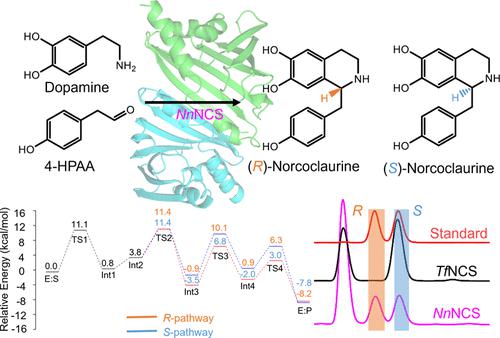
Benzylisoquinoline alkaloids (BIAs) are key plant metabolites that offer significant pharmacological benefits. While most naturally isolated BIAs are identified as (S)-enantiomers, (R)-configured BIAs are also abundant in specific species. However, the formation mechanism of (R)-enantiospecific BIAs remains largely unknown. Norcoclaurine synthase (NCS)-catalyzed Pictet–Spengler condensation is responsible for the BIAs scaffold formation and establishing a unique chiral carbon center. Nevertheless, all NCSs hitherto identified were strictly (S)-selective. Herein, five NCS homologues in lotus were identified and functionally characterized to be without enantiopreference, namely, being capable of producing (R)-norcoclaurine. We revealed the crystal structure of NnNCS1, one of the five identified NnNCS homologues, showing the enzyme’s typical pathogenesis-related protein 10-fold and an active dimeric form. Guided by the mechanistic information from quantum chemical calculations, the single-point mutation of Ile43, Leu60, and Phe101 leads to (R)-enantiospecific mutants. This study unravels the previously obscure pathway of (R)-BIA biosynthesis, thereby providing valuable enzymatic tools for the synthetic biology of (R)-configured BIAs.
中文翻译:

用于手性合成苄基异喹啉生物碱的 (R, S)-去甲月桂碱合酶的发现、结构和机制
苄基异喹啉生物碱 (BIA) 是重要的植物代谢物,具有显着的药理功效。虽然大多数天然分离的 BIA 被鉴定为 ( S )-对映体,但 ( R )-构型的 BIA 在特定物种中也很丰富。然而,( R )-对映特异性 BIA的形成机制仍然很大程度上未知。去甲乌云碱合酶 (NCS) 催化的 Pictet-Spengler 缩合负责 BIA 支架的形成并建立独特的手性碳中心。然而,迄今为止发现的所有 NCS 都是严格(S)选择性的。在此,鉴定了莲花中的五个NCS同源物,并进行了功能表征,其没有对映体优先性,即能够产生( R )-去甲乌云碱。我们揭示了Nn NCS1(五个已鉴定的Nn NCS 同源物之一)的晶体结构,显示了该酶典型的发病机制相关蛋白的 10 倍和活性二聚体形式。在量子化学计算的机制信息的指导下,Ile43、Leu60 和 Phe101 的单点突变导致 ( R )-对映特异性突变体。这项研究揭示了以前模糊的 ( R )-BIA 生物合成途径,从而为 ( R )-构型 BIA 的合成生物学提供了有价值的酶工具。
更新日期:2023-11-08
中文翻译:

用于手性合成苄基异喹啉生物碱的 (R, S)-去甲月桂碱合酶的发现、结构和机制
苄基异喹啉生物碱 (BIA) 是重要的植物代谢物,具有显着的药理功效。虽然大多数天然分离的 BIA 被鉴定为 ( S )-对映体,但 ( R )-构型的 BIA 在特定物种中也很丰富。然而,( R )-对映特异性 BIA的形成机制仍然很大程度上未知。去甲乌云碱合酶 (NCS) 催化的 Pictet-Spengler 缩合负责 BIA 支架的形成并建立独特的手性碳中心。然而,迄今为止发现的所有 NCS 都是严格(S)选择性的。在此,鉴定了莲花中的五个NCS同源物,并进行了功能表征,其没有对映体优先性,即能够产生( R )-去甲乌云碱。我们揭示了Nn NCS1(五个已鉴定的Nn NCS 同源物之一)的晶体结构,显示了该酶典型的发病机制相关蛋白的 10 倍和活性二聚体形式。在量子化学计算的机制信息的指导下,Ile43、Leu60 和 Phe101 的单点突变导致 ( R )-对映特异性突变体。这项研究揭示了以前模糊的 ( R )-BIA 生物合成途径,从而为 ( R )-构型 BIA 的合成生物学提供了有价值的酶工具。































 京公网安备 11010802027423号
京公网安备 11010802027423号