当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new synthesis of 3′-deoxy-3′-fluoroadenosine, a key intermediate of cyclic dinucleotide
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2023-11-05 , DOI: 10.1002/jhet.4751 Yayun Qiu 1 , Zixing Li 1 , Fangmin Ning 1 , Dandan Tang 1 , Huansheng Chen 1, 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2023-11-05 , DOI: 10.1002/jhet.4751 Yayun Qiu 1 , Zixing Li 1 , Fangmin Ning 1 , Dandan Tang 1 , Huansheng Chen 1, 2
Affiliation
|
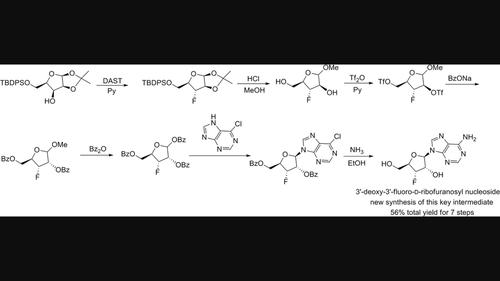
A new synthesis of 3′-deoxy-3′-fluoroadenosine for use as an important intermediate of antitumor-active cyclic dinucleotide is disclosed. The synthesis started with the known 5-O-TBDPS-D-lyxofuranose 1,2-acetonides, which was first transformed into a fluorinated compound after the DAST reaction. Desilylation and acidic methanolysis were then finished in one pot. After a sequence of triflation, displacement by OBz as well as benzoylation, perbenzoylated 3-deoxy-3-fluoro-D-ribofuranoside was obtained, which would be transformed to 3′-deoxy-3′-fluoroadenosine as the key intermediate of cyclic dinucleotide after ammonolysis.
中文翻译:

环状二核苷酸关键中间体3′-脱氧-3′-氟腺苷的新合成
公开了用作抗肿瘤活性环状二核苷酸的重要中间体的3'-脱氧-3'-氟腺苷的新合成。合成从已知的 5- O -TBDPS-D-呋喃来糖 1,2-丙酮化合物开始,在 DAST 反应后首先将其转化为氟化化合物。然后脱甲硅烷基化和酸性甲醇解在一锅中完成。经过一系列三氟甲磺酸化、OBz置换以及苯甲酰化,得到过苯甲酰化的3-脱氧-3-氟-D-呋喃核苷,将其转化为3'-脱氧-3'-氟腺苷,作为环状二核苷酸的关键中间体氨解后。
更新日期:2023-11-05
中文翻译:

环状二核苷酸关键中间体3′-脱氧-3′-氟腺苷的新合成
公开了用作抗肿瘤活性环状二核苷酸的重要中间体的3'-脱氧-3'-氟腺苷的新合成。合成从已知的 5- O -TBDPS-D-呋喃来糖 1,2-丙酮化合物开始,在 DAST 反应后首先将其转化为氟化化合物。然后脱甲硅烷基化和酸性甲醇解在一锅中完成。经过一系列三氟甲磺酸化、OBz置换以及苯甲酰化,得到过苯甲酰化的3-脱氧-3-氟-D-呋喃核苷,将其转化为3'-脱氧-3'-氟腺苷,作为环状二核苷酸的关键中间体氨解后。































 京公网安备 11010802027423号
京公网安备 11010802027423号