当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Detrimental Effects of Monoethanolamine and Other Amine-Based Capture Agents on the Electrochemical Reduction of CO2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2023-11-06 , DOI: 10.1021/acsenergylett.3c01953 John Safipour 1, 2 , Adam Z. Weber 2 , Alexis T. Bell 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2023-11-06 , DOI: 10.1021/acsenergylett.3c01953 John Safipour 1, 2 , Adam Z. Weber 2 , Alexis T. Bell 1, 2
Affiliation
|
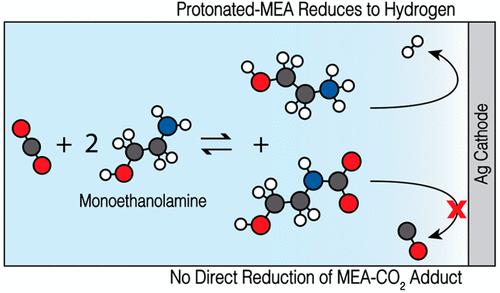
Reactive carbon capture (RCC) is a promising solution for carbon capture and utilization. RCC involves CO2 capture, typically with amine solutions to form carbamate, followed by immediate conversion into value-added chemicals and fuels, typically via electrochemical means. RCC may enhance CO2 reduction (CO2R) by overcoming the inherent limiting low solubility of CO2 in aqueous solutions. In this work, we present a systematic study of the influence of monoethanolamine (MEA) on CO2R performance over an Ag cathode in KHCO3 electrolytes. Contrary to prior work, the study finds no evidence for the direct reduction of carbamate anions. Instead, the presence of MEA suppresses the rate of CO formation while increasing that of H2. These results are supported by a boundary-layer continuum model of mass transport and reaction that correctly predicts experimental trends and demonstrates that MEA reduces the concentration of CO2 near the cathode. Thus, alternative strategies are necessary to achieve RCC in aqueous environments.
中文翻译:

单乙醇胺和其他胺基捕获剂对 CO2 电化学还原的不利影响
反应性碳捕获(RCC)是碳捕获和利用的一种有前途的解决方案。RCC 涉及 CO 2捕获,通常使用胺溶液形成氨基甲酸盐,然后通常通过电化学方式立即转化为增值化学品和燃料。RCC可以通过克服CO 2在水溶液中固有的限制性低溶解度来增强CO 2还原(CO 2 R)。在这项工作中,我们系统地研究了单乙醇胺 (MEA) 对KHCO 3电解质中银阴极上的 CO 2 R 性能的影响。与之前的工作相反,该研究没有发现直接减少氨基甲酸盐阴离子的证据。相反,MEA 的存在抑制了 CO 的形成速率,同时增加了 H 2的形成速率。这些结果得到了质量传递和反应的边界层连续模型的支持,该模型正确预测了实验趋势,并证明 MEA 降低了阴极附近的 CO 2浓度。因此,需要替代策略来在水环境中实现 RCC。
更新日期:2023-11-06
中文翻译:

单乙醇胺和其他胺基捕获剂对 CO2 电化学还原的不利影响
反应性碳捕获(RCC)是碳捕获和利用的一种有前途的解决方案。RCC 涉及 CO 2捕获,通常使用胺溶液形成氨基甲酸盐,然后通常通过电化学方式立即转化为增值化学品和燃料。RCC可以通过克服CO 2在水溶液中固有的限制性低溶解度来增强CO 2还原(CO 2 R)。在这项工作中,我们系统地研究了单乙醇胺 (MEA) 对KHCO 3电解质中银阴极上的 CO 2 R 性能的影响。与之前的工作相反,该研究没有发现直接减少氨基甲酸盐阴离子的证据。相反,MEA 的存在抑制了 CO 的形成速率,同时增加了 H 2的形成速率。这些结果得到了质量传递和反应的边界层连续模型的支持,该模型正确预测了实验趋势,并证明 MEA 降低了阴极附近的 CO 2浓度。因此,需要替代策略来在水环境中实现 RCC。





























 京公网安备 11010802027423号
京公网安备 11010802027423号