当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antioxidant Activity and Skin Sensitization of Eugenol and Isoeugenol: Two Sides of the Same Coin?
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2023-11-03 , DOI: 10.1021/acs.chemrestox.3c00263 Yannick Port-Lougarre 1 , Christophe Gourlaouen 1 , Bertrand Vileno 1 , Elena Giménez-Arnau 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2023-11-03 , DOI: 10.1021/acs.chemrestox.3c00263 Yannick Port-Lougarre 1 , Christophe Gourlaouen 1 , Bertrand Vileno 1 , Elena Giménez-Arnau 1
Affiliation
|
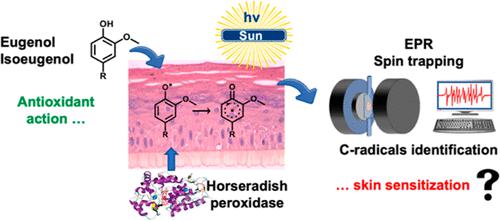
Eugenol and isoeugenol are well acknowledged to possess antioxidant and thus cytoprotective activities. Yet both compounds are also important skin sensitizers, compelling the cosmetics and fragrance industries to notify their presence in manufactured products. While they are structurally very similar, they show significant differences in their sensitization properties. Consequently, eugenol and isoeugenol have been the subject of many mechanistic studies where the final oxidation forms, electrophilic ortho-quinone and quinone methide, are blamed as the reactive species forming an antigenic complex with nucleophilic residues of skin proteins, inducing skin sensitization. However, radical mechanisms could compete with such an electrophilic-nucleophilic pathway. The antioxidant activity results from neutralizing reactive oxygen radicals by the release of the phenolic hydrogen atom. The so-formed phenoxyl radicals can then fully delocalize upon the structure, becoming potentially reactive toward skin proteins at several positions. To obtain in-depth insights into such reactivity, we investigated in situ the formation of radicals from eugenol and isoeugenol using electron paramagnetic resonance combined with spin trapping in reconstructed human epidermis (RHE), mimicking human skin and closer to what may happen in vivo. Two modes of radical initiation were used, exposing RHE to (i) horseradish peroxidase (HRP), complementing RHE metabolic capacities, and mimicking peroxidases present in vivo or (ii) solar light using a AM 1.5 solar simulator. In both experimental approaches, where the antioxidant character of both compounds is revealed, oxygen- and carbon-centered radicals were formed in RHE. Our hypothesis is that such carbon radicals are relevant candidates to form antigenic entities prior to conversion into electrophilic quinones. On this basis, these studies suggest that pro- or prehapten fingerprints could be advanced depending on the radical initiation method. The introduction of HRP suggested that eugenol and isoeugenol behave as prohaptens, while when exposed to light, a prehapten nature could be highlighted.
中文翻译:

丁子香酚和异丁子香酚的抗氧化活性和皮肤过敏:同一枚硬币的两面?
众所周知,丁子香酚和异丁子香酚具有抗氧化作用,因此具有细胞保护活性。然而,这两种化合物也是重要的皮肤致敏剂,迫使化妆品和香水行业告知其在制成品中的存在。虽然它们在结构上非常相似,但它们的敏化特性却表现出显着差异。因此,丁子香酚和异丁子香酚已成为许多机制研究的主题,其中最终氧化形式亲电邻醌和醌甲基化物被认为是与皮肤蛋白质的亲核残基形成抗原复合物的反应性物质,从而诱导皮肤过敏。然而,激进的机制可能会与这种亲电-亲核途径竞争。抗氧化活性是通过释放酚氢原子中和活性氧自由基而产生的。如此形成的苯氧基自由基可以在结构上完全离域,在多个位置对皮肤蛋白产生潜在的反应。为了深入了解这种反应性,我们利用电子顺磁共振结合重建人表皮(RHE)中的自旋捕获,原位研究了丁子香酚和异丁子香酚自由基的形成,模仿人类皮肤,更接近体内可能发生的情况。使用两种自由基引发模式,将 RHE 暴露于 (i) 辣根过氧化物酶 (HRP),补充 RHE 代谢能力,并模仿体内存在的过氧化物酶或 (ii) 使用 AM 1.5 太阳模拟器的太阳光。在两种实验方法中,两种化合物的抗氧化特性都被揭示,RHE 中形成了以氧和碳为中心的自由基。 我们的假设是,此类碳自由基是在转化为亲电子醌之前形成抗原实体的相关候选者。在此基础上,这些研究表明,前半抗原或前半抗原指纹可以根据自由基引发方法进行改进。 HRP 的引入表明丁子香酚和异丁子香酚表现为前半抗原,而当暴露在光下时,前半抗原的性质可能会突出。
更新日期:2023-11-03
中文翻译:

丁子香酚和异丁子香酚的抗氧化活性和皮肤过敏:同一枚硬币的两面?
众所周知,丁子香酚和异丁子香酚具有抗氧化作用,因此具有细胞保护活性。然而,这两种化合物也是重要的皮肤致敏剂,迫使化妆品和香水行业告知其在制成品中的存在。虽然它们在结构上非常相似,但它们的敏化特性却表现出显着差异。因此,丁子香酚和异丁子香酚已成为许多机制研究的主题,其中最终氧化形式亲电邻醌和醌甲基化物被认为是与皮肤蛋白质的亲核残基形成抗原复合物的反应性物质,从而诱导皮肤过敏。然而,激进的机制可能会与这种亲电-亲核途径竞争。抗氧化活性是通过释放酚氢原子中和活性氧自由基而产生的。如此形成的苯氧基自由基可以在结构上完全离域,在多个位置对皮肤蛋白产生潜在的反应。为了深入了解这种反应性,我们利用电子顺磁共振结合重建人表皮(RHE)中的自旋捕获,原位研究了丁子香酚和异丁子香酚自由基的形成,模仿人类皮肤,更接近体内可能发生的情况。使用两种自由基引发模式,将 RHE 暴露于 (i) 辣根过氧化物酶 (HRP),补充 RHE 代谢能力,并模仿体内存在的过氧化物酶或 (ii) 使用 AM 1.5 太阳模拟器的太阳光。在两种实验方法中,两种化合物的抗氧化特性都被揭示,RHE 中形成了以氧和碳为中心的自由基。 我们的假设是,此类碳自由基是在转化为亲电子醌之前形成抗原实体的相关候选者。在此基础上,这些研究表明,前半抗原或前半抗原指纹可以根据自由基引发方法进行改进。 HRP 的引入表明丁子香酚和异丁子香酚表现为前半抗原,而当暴露在光下时,前半抗原的性质可能会突出。































 京公网安备 11010802027423号
京公网安备 11010802027423号