当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrophotochemical Synthesis Facilitated Trifluoromethylation of Arenes Using Trifluoroacetic Acid
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-03 , DOI: 10.1021/jacs.3c10148 Jing Qi 1 , Jinhui Xu 1 , Hwee Ting Ang 1 , Bingbing Wang 1 , Nipun Kumar Gupta 2 , Srinivas Reddy Dubbaka 3 , Patrick O'Neill 4 , Xianwen Mao 5 , Yanwei Lum 6 , Jie Wu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-03 , DOI: 10.1021/jacs.3c10148 Jing Qi 1 , Jinhui Xu 1 , Hwee Ting Ang 1 , Bingbing Wang 1 , Nipun Kumar Gupta 2 , Srinivas Reddy Dubbaka 3 , Patrick O'Neill 4 , Xianwen Mao 5 , Yanwei Lum 6 , Jie Wu 1
Affiliation
|
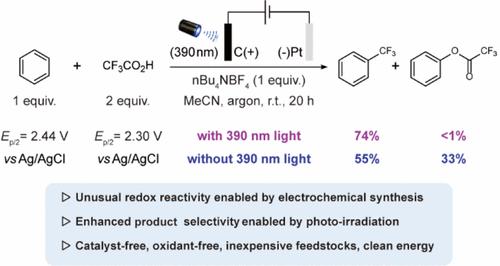
The trifluoromethyl (CF3) group is an essential moiety in medicinal chemistry due to its unique physicochemical properties. While trifluoroacetic acid (TFA) is an inexpensive and easily accessible reagent, its use as a source of CF3 is highly challenging due to its high oxidation potential. In this study, we present a novel electrophotochemical approach that enables the use of TFA as the CF3 source for the selective, catalyst- and oxidant-free trifluoromethylation of (hetero)arenes. Key to our approach is the selective oxidation of TFA over arenes, generating CF3 radicals through oxidative decarboxylation. This strategy enables the sustainable and environmentally-friendly synthesis of CF3-, CF2H- and perfluoroalkyl-containing (hetero)arenes with a broad range of substrates. Importantly, our results demonstrate significantly improved chemoselectivity by light irradiation, opening up new possibilities for the synthetic and medicinal applications of TFA as an ideal yet underutilized CF3 source.
中文翻译:

使用三氟乙酸的电化学合成促进芳烃的三氟甲基化
三氟甲基(CF 3)基团因其独特的理化性质而成为药物化学中的重要基团。虽然三氟乙酸 (TFA) 是一种廉价且易于获得的试剂,但由于其高氧化电位,将其用作 CF 3来源极具挑战性。在这项研究中,我们提出了一种新颖的电光化学方法,该方法能够使用TFA作为CF 3源,对(杂)芳烃进行选择性、无催化剂和无氧化剂的三氟甲基化。我们方法的关键是 TFA 对芳烃的选择性氧化,通过氧化脱羧产生 CF 3自由基。该策略能够以多种底物可持续且环保地合成CF 3 -、CF 2 H-和含全氟烷基的(杂)芳烃。重要的是,我们的结果表明光照射显着提高了化学选择性,为 TFA 作为理想但未充分利用的 CF 3源的合成和医学应用开辟了新的可能性。
更新日期:2023-11-03
中文翻译:

使用三氟乙酸的电化学合成促进芳烃的三氟甲基化
三氟甲基(CF 3)基团因其独特的理化性质而成为药物化学中的重要基团。虽然三氟乙酸 (TFA) 是一种廉价且易于获得的试剂,但由于其高氧化电位,将其用作 CF 3来源极具挑战性。在这项研究中,我们提出了一种新颖的电光化学方法,该方法能够使用TFA作为CF 3源,对(杂)芳烃进行选择性、无催化剂和无氧化剂的三氟甲基化。我们方法的关键是 TFA 对芳烃的选择性氧化,通过氧化脱羧产生 CF 3自由基。该策略能够以多种底物可持续且环保地合成CF 3 -、CF 2 H-和含全氟烷基的(杂)芳烃。重要的是,我们的结果表明光照射显着提高了化学选择性,为 TFA 作为理想但未充分利用的 CF 3源的合成和医学应用开辟了新的可能性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号