European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-11-03 , DOI: 10.1016/j.ejmech.2023.115920 Qianqian Feng 1 , Jinfeng Zhang 1 , Shuang Luo 1 , Yong Huang 2 , Zhiyun Peng 3 , Guangcheng Wang 4
|
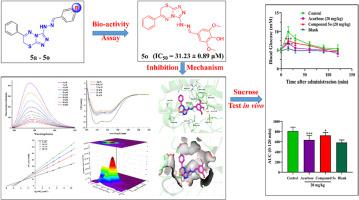
In our work, several 7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine-phenylhydrazone derivatives as α-glucosidase inhibitors (α-GIs) were synthesized and characterized by 1H NMR, 13C NMR, and HRMS spectrum. Then, their bio-activity against the α-glucosidase (α-Glu) was further evaluated. Among them, almost all compounds displayed better bio-activity with IC50 from 31.23 ± 0.89 to 213.50 ± 4.19 μM than acarbose (IC50 = 700.20 ± 10.55 μM). In particular, compound 5o showed the best potency to inhibit α-Glu in a mixed manner. Moreover, the action mechanisms of 5o were further clarified including fluorescence quenching, circular dichroism spectra, three-dimensional fluorescence spectra, molecular docking, etc. All mechanism studies revealed that 5o could arouse the changed secondary structure of α-Glu to hinder enzyme catalytic activity. It was observed from an in vivo study that 5o of 20 mg/kg could significantly decrease by 24.45 % postprandial blood glucose in mice vs. the control. Meanwhile, 5o had low drug-drug interaction potential and was likely to be an orally active compound. Moreover, 5o was observed to be no obvious cytotoxicity to HEK-293 cells. In summary, compound 5o exhibited one potential to be further applied as an antidiabetic drug.
中文翻译:

α-葡萄糖苷酶抑制剂7H-[1,2,4]三唑并[3,4-b][1,3,4]噻二嗪苯腙衍生物的合成、生物学评价及作用机制
在我们的工作中,合成了几种作为 α-葡萄糖苷酶抑制剂 (α-GI) 的7H -[1,2,4]三唑并[3,4-b][1,3,4]噻二嗪-苯腙衍生物,并通过1 H进行表征。 NMR、13 C NMR 和 HRMS 谱。然后,进一步评估了它们针对α-葡萄糖苷酶(α-Glu)的生物活性。其中,几乎所有化合物都表现出比阿卡波糖更好的生物活性,IC 50为 31.23 ± 0.89 至 213.50 ± 4.19 μM(IC 50 = 700.20 ± 10.55 μM)。特别是,化合物5o显示出以混合方式抑制 α-Glu 的最佳效力。此外,进一步阐明了5o的作用机制,包括荧光猝灭、圆二色光谱、三维荧光光谱、分子对接等。所有机制研究均表明5o可以引起α-Glu二级结构的改变,阻碍酶的催化活性。 。体内研究发现, 5o 20 mg/kg 可使小鼠餐后血糖较对照组显着降低 24.45%。同时,5o具有较低的药物相互作用潜力,并且可能是一种口服活性化合物。而且,观察到5o对HEK-293细胞没有明显的细胞毒性。总之,化合物5o表现出进一步作为抗糖尿病药物应用的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号