当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Progress in the Treatment of Peritoneal Metastatic Cancer and the Application of Therapeutic Nanoagents
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2023-11-02 , DOI: 10.1021/acsabm.3c00662
Wenjun Dai 1 , Yidan Chen 2 , Yunxin Xue 1 , Mimi Wan 1 , Chun Mao 1 , Ke Zhang 2
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2023-11-02 , DOI: 10.1021/acsabm.3c00662
Wenjun Dai 1 , Yidan Chen 2 , Yunxin Xue 1 , Mimi Wan 1 , Chun Mao 1 , Ke Zhang 2
Affiliation
|
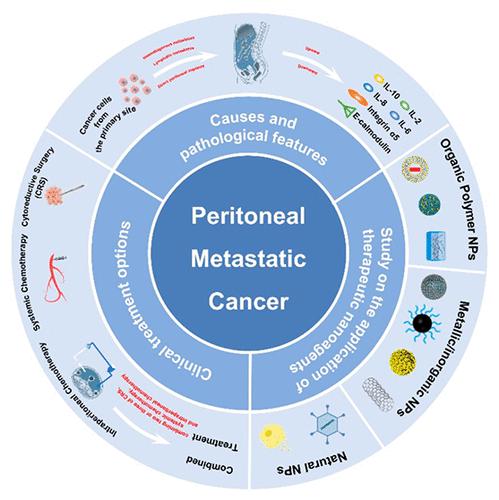
Peritoneal metastatic cancer is a cancer caused by the direct growth of cancer cells from the primary site through the bloodstream, lymph, or peritoneum, which is a difficult part of current clinical treatment. In the abdominal cavity of patients with metastatic peritoneal cancer, there are usually nodules of various sizes and malignant ascites. Among them, nodules of different sizes can obstruct intestinal movement and form intestinal obstruction, while malignant ascites can cause abdominal distension and discomfort, and even cause patients to have difficulty in breathing. The pathology and physiology of peritoneal metastatic cancer are complex and not fully understood. The main hypothesis is “seed” and “soil”; i.e., cells from the primary tumor are shed and implanted in the peritoneal cavity (peritoneal metastasis). In the last two decades, the main treatment modalities used clinically are cytoreductive surgery (CRS), systemic chemotherapy, intraperitoneal chemotherapy, and combined treatment, all of which help to improve patient survival and quality of life (QOL). However, the small-molecule chemotherapeutic drugs used clinically still have problems such as rapid drug metabolism and systemic toxicity. With the rapid development of nanotechnology in recent years, therapeutic nanoagents for the treatment of peritoneal metastatic cancer have been gradually developed, which has improved the therapeutic effect and reduced the systemic toxicity of small-molecule chemotherapeutic drugs to a certain extent. In addition, nanomaterials have been developed not only as therapeutic agents but also as imaging agents to guide peritoneal tumor CRS. In this review, we describe the etiology and pathological features of peritoneal metastatic cancer, discuss in detail the clinical treatments that have been used for peritoneal metastatic cancer, and analyze the advantages and disadvantages of the different clinical treatments and the QOL of the treated patients, followed by a discussion focusing on the progress, obstacles, and challenges in the use of therapeutic nanoagents in peritoneal metastatic cancer. Finally, therapeutic nanoagents and therapeutic tools that may be used in the future for the treatment of peritoneal metastatic cancer are prospected.
中文翻译:

腹膜转移癌治疗及纳米药物应用进展
腹膜转移癌是癌细胞从原发部位通过血流、淋巴液或腹膜直接生长而引起的癌症,是目前临床治疗的难点。转移性腹膜癌患者的腹腔内通常存在大小不一的结节和恶性腹水。其中,大小不等的结节可阻碍肠道运动,形成肠梗阻,而恶性腹水则可引起腹胀不适,甚至导致患者呼吸困难。腹膜转移癌的病理学和生理学很复杂且尚未完全了解。主要假设是“种子”和“土壤”;即,原发肿瘤的细胞脱落并植入腹膜腔(腹膜转移)。近二十年来,临床上使用的主要治疗方式是细胞减灭术(CRS)、全身化疗、腹腔化疗和联合治疗,这些都有助于提高患者的生存率和生活质量(QOL)。但临床使用的小分子化疗药物仍存在药物代谢快、全身毒性等问题。近年来,随着纳米技术的快速发展,用于治疗腹膜转移癌的治疗性纳米药物逐渐被开发出来,在一定程度上提高了治疗效果并降低了小分子化疗药物的全身毒性。此外,纳米材料不仅被开发作为治疗剂,而且还被开发作为引导腹膜肿瘤CRS的成像剂。 在这篇综述中,我们描述了腹膜转移癌的病因和病理特征,详细讨论了腹膜转移癌的临床治疗方法,并分析了不同临床治疗方法的优缺点以及治疗患者的生活质量。接下来的讨论重点是使用治疗性纳米药物治疗腹膜转移癌的进展、障碍和挑战。最后,展望了未来可能用于治疗腹膜转移癌的治疗纳米药物和治疗工具。
更新日期:2023-11-02
中文翻译:

腹膜转移癌治疗及纳米药物应用进展
腹膜转移癌是癌细胞从原发部位通过血流、淋巴液或腹膜直接生长而引起的癌症,是目前临床治疗的难点。转移性腹膜癌患者的腹腔内通常存在大小不一的结节和恶性腹水。其中,大小不等的结节可阻碍肠道运动,形成肠梗阻,而恶性腹水则可引起腹胀不适,甚至导致患者呼吸困难。腹膜转移癌的病理学和生理学很复杂且尚未完全了解。主要假设是“种子”和“土壤”;即,原发肿瘤的细胞脱落并植入腹膜腔(腹膜转移)。近二十年来,临床上使用的主要治疗方式是细胞减灭术(CRS)、全身化疗、腹腔化疗和联合治疗,这些都有助于提高患者的生存率和生活质量(QOL)。但临床使用的小分子化疗药物仍存在药物代谢快、全身毒性等问题。近年来,随着纳米技术的快速发展,用于治疗腹膜转移癌的治疗性纳米药物逐渐被开发出来,在一定程度上提高了治疗效果并降低了小分子化疗药物的全身毒性。此外,纳米材料不仅被开发作为治疗剂,而且还被开发作为引导腹膜肿瘤CRS的成像剂。 在这篇综述中,我们描述了腹膜转移癌的病因和病理特征,详细讨论了腹膜转移癌的临床治疗方法,并分析了不同临床治疗方法的优缺点以及治疗患者的生活质量。接下来的讨论重点是使用治疗性纳米药物治疗腹膜转移癌的进展、障碍和挑战。最后,展望了未来可能用于治疗腹膜转移癌的治疗纳米药物和治疗工具。

































 京公网安备 11010802027423号
京公网安备 11010802027423号