Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2023-11-03 , DOI: 10.1016/j.jfluchem.2023.110215 Bohdan Moroz , Kostiantyn P. Melnykov , Serhii Holovach , Andrey A. Filatov , Oleksii Raievskyi , Maksym Platonov , Oleksandr Liashuk , Dmytro M. Volochnyuk , Oleksandr O. Grygorenko
|
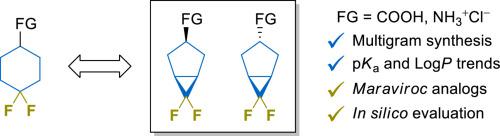
Multigram synthesis of diastereomerically pure cis- and trans-6,6-difluorobicyclo[3.1.0]hexane building blocks starting from commercially available compounds is described. The diastereomeric mixture obtained by reaction of TMSCF3 – NaI system with non-activated cyclopentene fragment using the slow addition protocol was effectively separated by flash column chromatography. Further simple chemical transformations produced diastereopure amines and carboxylic acids – promising building blocks for drug discovery. Physicochemical properties (i.e., pKa and LogP) were measured for the title scaffold derivatives and compared with those with monocyclic and non-fluorinated counterparts. Two rigidified analogues of marketed anti-HIV drug Maraviroc were synthesized and evaluated by docking and molecular dynamics studies.
中文翻译:

6,6-二氟双环[3.1.0]己烷作为刚性 4,4-二氟环己烷模拟物:多克合成、物理化学表征以及并入 Maraviroc 类似物
描述了从市售化合物开始非对映体纯的顺式和反式-6,6-二氟双环[3.1.0]己烷结构单元的多克合成。TMSCF 3 – NaI 体系与非活化环戊烯片段采用缓慢添加方案反应获得的非对映异构体混合物可通过快速柱色谱有效分离。进一步简单的化学转化产生了非对映纯胺和羧酸——有希望成为药物发现的基础材料。测量了标题支架衍生物的物理化学性质(即pKa和LogP),并与单环和非氟化对应物进行比较。通过对接和分子动力学研究合成并评估了市售抗 HIV 药物 Maraviroc 的两种刚性类似物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号