Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2023-11-03 , DOI: 10.1016/j.bmc.2023.117516 Izadora Amaral Nakao 1 , Tamires Cunha Almeida 1 , Adriana Cotta Cardoso Reis 1 , Gabrielly Guimarães Coutinho 1 , Aline Mol Hermenegildo 1 , Cleydson Finotti Cordeiro 2 , Glenda Nicioli da Silva 1 , Danielle Ferreira Dias 3 , Geraldo Célio Brandão 1 , Saulo Fehelberg Pinto Braga 1 , Thiago Belarmino de Souza 1
|
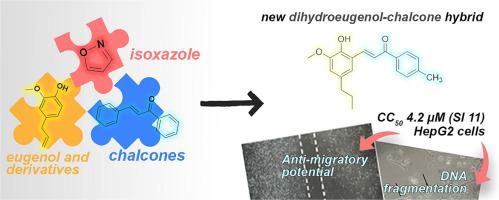
Cancer still represents a serious public health problem and one of the main problems related to the worsening of this disease is the ability of some tumors to develop metastasis. In this work, we synthesized a new series of chalcones and isoxazoles derived from eugenol and analogues as molecular hybrids and these compounds were evaluated against different tumor cell lines. This structural pattern was designed considering the cytotoxic potential already known for eugenol, chalcones and isoxazoles. Notably, chalcones 7, 9, 10, and 11 displayed significant activity (4.2–14.5 µM) against two cancer cell lines, surpassing the potency of the control drug doxorubicin. The reaction of chalcones with hydroxylamine hydrochloride provided the corresponding isoxazoles that were inactive against these cancer cells. The dihydroeugenol chalcone 7 showed the most promising results, demonstrating higher potency against HepG2 (CC50: 4.2 µM) and TOV-21G (CC50: 7.2 µM). Chalcone 7 was also three times less toxic than doxorubicin considering HepG2 cells, with a selectivity index greater than 11. Further investigations including clonogenic survival, cell cycle progression and cell migration assays confirmed the compelling antitumoral potential of chalcone 7, as it reduced long-term survival due to DNA fragmentation, inducing cell death and inhibiting HepG2 cells migration. Moreover, in silico studies involving docking and molecular dynamics revealed a consistent binding mode of chalcone 7 with metalloproteinases, particularly MMP-9, shedding light on its potential mechanism of action related to anti-migratory effects. These significant findings suggest the inclusion of compound 7 as a promising candidate for future studies in the field of cancer therapeutics.
中文翻译:

发现一种具有细胞毒性和抗迁移潜力的新型二氢丁香酚-查尔酮杂合体:癌症治疗的双重作用
癌症仍然是一个严重的公共卫生问题,与这种疾病恶化相关的主要问题之一是某些肿瘤发生转移的能力。在这项工作中,我们合成了一系列新的源自丁子香酚和类似物的查耳酮和异恶唑作为分子杂合体,并针对不同的肿瘤细胞系评估了这些化合物。这种结构模式的设计考虑了已知的丁子香酚、查耳酮和异恶唑的细胞毒性潜力。值得注意的是,查尔酮7 、 9 、 10和11对两种癌细胞系表现出显着的活性(4.2–14.5 µM),超过了对照药物阿霉素的效力。查耳酮与盐酸羟胺的反应提供了相应的异恶唑,对这些癌细胞没有活性。二氢丁香酚查耳酮7显示出最有希望的结果,对 HepG2 (CC 50 : 4.2 µM) 和 TOV-21G (CC 50 : 7.2 µM) 具有更高的效力。考虑到 HepG2 细胞,查耳酮7 的毒性也比多柔比星低三倍,选择性指数大于 11。包括克隆形成存活、细胞周期进展和细胞迁移测定在内的进一步研究证实了查耳酮7令人信服的抗肿瘤潜力,因为它长期减少DNA 碎片导致细胞存活,诱导细胞死亡并抑制 HepG2 细胞迁移。 此外,涉及对接和分子动力学的计算机研究揭示了查尔酮7与金属蛋白酶(特别是 MMP-9)一致的结合模式,揭示了其与抗迁移作用相关的潜在作用机制。这些重要的发现表明化合物7可以作为癌症治疗领域未来研究的有希望的候选者。































 京公网安备 11010802027423号
京公网安备 11010802027423号