当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calculation of Vibrational Shifts of Nitrile Probes in the Active Site of Ketosteroid Isomerase upon Ligand Binding
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-12-31 , DOI: 10.1021/ja3084384 Joshua P Layfield 1 , Sharon Hammes-Schiffer
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-12-31 , DOI: 10.1021/ja3084384 Joshua P Layfield 1 , Sharon Hammes-Schiffer
Affiliation
|
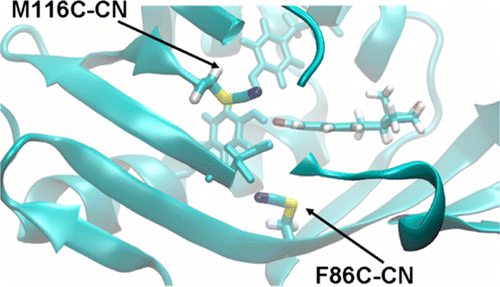
The vibrational Stark effect provides insight into the roles of hydrogen bonding, electrostatics, and conformational motions in enzyme catalysis. In a recent application of this approach to the enzyme ketosteroid isomerase (KSI), thiocyanate probes were introduced in site-specific positions throughout the active site. This paper implements a quantum mechanical/molecular mechanical (QM/MM) approach for calculating the vibrational shifts of nitrile (CN) probes in proteins. This methodology is shown to reproduce the experimentally measured vibrational shifts upon binding of the intermediate analogue equilinen to KSI for two different nitrile probe positions. Analysis of the molecular dynamics simulations provides atomistic insight into the roles that key residues play in determining the electrostatic environment and hydrogen-bonding interactions experienced by the nitrile probe. For the M116C-CN probe, equilinen binding reorients an active-site water molecule that is directly hydrogen-bonded to the nitrile probe, resulting in a more linear C≡N--H angle and increasing the CN frequency upon binding. For the F86C-CN probe, equilinen binding orients the Asp103 residue, decreasing the hydrogen-bonding distance between the Asp103 backbone and the nitrile probe and slightly increasing the CN frequency. This QM/MM methodology is applicable to a wide range of biological systems and has the potential to assist in the elucidation of the fundamental principles underlying enzyme catalysis.
中文翻译:

计算配体结合后酮类固醇异构酶活性位点中腈探针的振动位移
振动斯塔克效应提供了对氢键、静电和构象运动在酶催化中的作用的深入了解。在最近将这种方法应用于酶酮类固醇异构酶 (KSI) 中,硫氰酸盐探针被引入到整个活性位点的位点特定位置。本文采用量子力学/分子力学 (QM/MM) 方法来计算蛋白质中腈 (CN) 探针的振动位移。该方法显示在中间类似物 equilinen 与两个不同腈探针位置的 KSI 结合后重现实验测量的振动位移。分子动力学模拟分析提供了对关键残基在确定腈探针经历的静电环境和氢键相互作用中所起作用的原子洞察。对于 M116C-CN 探针,equilinen 结合重新定向与腈探针直接氢键连接的活性位点水分子,从而产生更线性的 C≡N--H 角并在结合时增加 CN 频率。对于 F86C-CN 探针,equilinen 结合定向 Asp103 残基,减少 Asp103 骨架和腈探针之间的氢键距离并略微增加 CN 频率。这种 QM/MM 方法适用于广泛的生物系统,并有可能有助于阐明酶催化的基本原理。
更新日期:2012-12-31
中文翻译:

计算配体结合后酮类固醇异构酶活性位点中腈探针的振动位移
振动斯塔克效应提供了对氢键、静电和构象运动在酶催化中的作用的深入了解。在最近将这种方法应用于酶酮类固醇异构酶 (KSI) 中,硫氰酸盐探针被引入到整个活性位点的位点特定位置。本文采用量子力学/分子力学 (QM/MM) 方法来计算蛋白质中腈 (CN) 探针的振动位移。该方法显示在中间类似物 equilinen 与两个不同腈探针位置的 KSI 结合后重现实验测量的振动位移。分子动力学模拟分析提供了对关键残基在确定腈探针经历的静电环境和氢键相互作用中所起作用的原子洞察。对于 M116C-CN 探针,equilinen 结合重新定向与腈探针直接氢键连接的活性位点水分子,从而产生更线性的 C≡N--H 角并在结合时增加 CN 频率。对于 F86C-CN 探针,equilinen 结合定向 Asp103 残基,减少 Asp103 骨架和腈探针之间的氢键距离并略微增加 CN 频率。这种 QM/MM 方法适用于广泛的生物系统,并有可能有助于阐明酶催化的基本原理。































 京公网安备 11010802027423号
京公网安备 11010802027423号