当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kilogram-Scale Preparation of the Amino Alcohol Fragment of Selgantolimod by Enzymatic Resolution of an α,α-Disubstituted Amino Ester
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-11-02 , DOI: 10.1021/acs.oprd.3c00271 Adam B. Weinstein 1 , Florence J. Bachrach 1 , Amy Cagulada 1 , Christopher S. Regens 1 , Peng Zhang 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-11-02 , DOI: 10.1021/acs.oprd.3c00271 Adam B. Weinstein 1 , Florence J. Bachrach 1 , Amy Cagulada 1 , Christopher S. Regens 1 , Peng Zhang 2
Affiliation
|
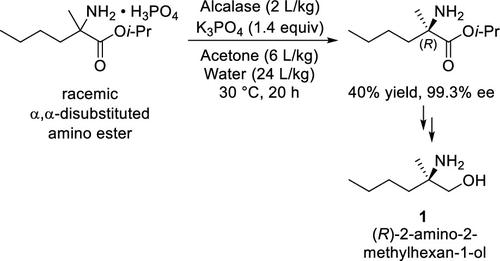
The chiral amino alcohol (R)-2-amino-2-methylhexan-1-ol (1) is a key fragment in the synthesis of selgantolimod, a TLR8 agonist that is being evaluated for the treatment of hepatitis B infection. This report describes the development of a robust and scalable synthesis of the targeted amino alcohol featuring a hydrolase-catalyzed kinetic resolution of an α,α-disubstituted amino ester. The results highlight considerations for substrate design for the enzymatic resolution, the impact of pH on the resolution of an unprotected α,α-disubstituted amino ester derivative, and implementation of this substrate within a route to the desired amino alcohol fragment.
中文翻译:

通过酶法拆分 α,α-二取代氨基酯公斤级制备 Selgantolimod 氨基醇片段
手性氨基醇 ( R )-2-氨基-2-甲基己-1-醇 ( 1 ) 是合成 selgantolimod 的关键片段,selgantolimod 是一种 TLR8 激动剂,正在评估其治疗乙型肝炎感染的效果。该报告描述了目标氨基醇的稳健且可扩展的合成方法的开发,其特点是水解酶催化的α,α-二取代氨基酯的动力学拆分。结果强调了酶促拆分底物设计的考虑因素、pH 对未受保护的 α,α-二取代氨基酯衍生物拆分的影响,以及该底物在获得所需氨基醇片段的途径中的实现。
更新日期:2023-11-02
中文翻译:

通过酶法拆分 α,α-二取代氨基酯公斤级制备 Selgantolimod 氨基醇片段
手性氨基醇 ( R )-2-氨基-2-甲基己-1-醇 ( 1 ) 是合成 selgantolimod 的关键片段,selgantolimod 是一种 TLR8 激动剂,正在评估其治疗乙型肝炎感染的效果。该报告描述了目标氨基醇的稳健且可扩展的合成方法的开发,其特点是水解酶催化的α,α-二取代氨基酯的动力学拆分。结果强调了酶促拆分底物设计的考虑因素、pH 对未受保护的 α,α-二取代氨基酯衍生物拆分的影响,以及该底物在获得所需氨基醇片段的途径中的实现。































 京公网安备 11010802027423号
京公网安备 11010802027423号