当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Pyrazole-5-yl-amide Derivatives Containing Cinnamamide Structural Fragments as Potential Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-11-03 , DOI: 10.1021/acs.jafc.3c04355
Xiang Cheng 1 , Zonghan Xu 2 , Hongyun Cui 2 , Zhen Zhang 2 , Wei Chen 2 , Fanglei Wang 1 , Shanlu Li 2 , Qixuan Liu 2 , Dandan Wang 2 , Xianhai Lv 1, 2 , Xihao Chang 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-11-03 , DOI: 10.1021/acs.jafc.3c04355
Xiang Cheng 1 , Zonghan Xu 2 , Hongyun Cui 2 , Zhen Zhang 2 , Wei Chen 2 , Fanglei Wang 1 , Shanlu Li 2 , Qixuan Liu 2 , Dandan Wang 2 , Xianhai Lv 1, 2 , Xihao Chang 1, 2
Affiliation
|
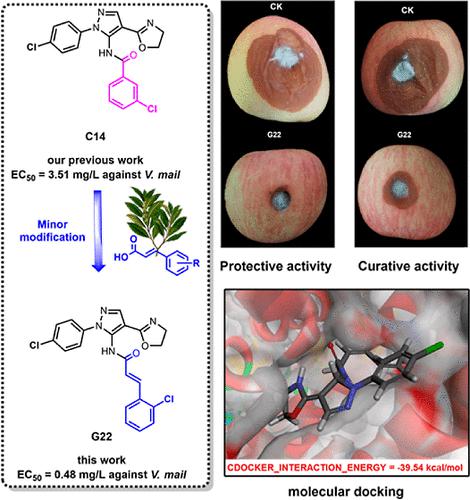
To promote the development of novel agricultural succinate dehydrogenase inhibitor (SDHI) fungicides, we introduced cinnamamide and nicotinamide structural fragments into the structure of pyrazol-5-yl-amide by carbon chain extension and scaffold hopping, respectively, and synthesized a series of derivatives. The results of the biological activity assays indicated that most of the target compounds exhibited varying degrees of inhibitory activity against the tested fungi. Notably, compounds G22, G28, G34, G38, and G39 exhibited excellent in vitro antifungal activities against Valsa mali with EC50 values of 0.48, 0.86, 0.57, 0.73, and 0.87 mg/L, respectively, and this result was significantly more potent than boscalid (EC50 = 2.80 mg/L) and closer to the specialty control drug tebuconazole (EC50 = 0.30 mg/L). Compounds G22 and G34 also exhibited excellent in vivo protective and curative effects against V. mali at 40 mg/L. The SEM and TEM observations indicated that compounds G22 and G34 may affect normal V. mali mycelial morphology as well as the cellular ultrastructure. Molecular docking analysis results indicated that G22 and boscalid possessed a similar binding mode to that of SDH, and detailed SDH inhibition assays validated the feasibility of the designed compounds as potential SDH inhibitors. Compounds G22 and G3 were selected for theoretical calculations, and the terminal carboxylic acid group of this series of compounds may be a key region influencing the antifungal activity. Furthermore, toxicity tests on Apis mellifera l. revealed that compounds G22 and G34 exhibited low toxicity to A. mellifera l. populations. The above results demonstrated that these series of pyrazole-5-yl-amide derivatives are promising for development as potential low-risk drug-resistance agricultural SDHI fungicides.
中文翻译:

含有肉桂酰胺结构片段的吡唑-5-基-酰胺衍生物作为潜在琥珀酸脱氢酶抑制剂的发现
为了促进新型农用琥珀酸脱氢酶抑制剂(SDHI)杀菌剂的开发,我们通过碳链延伸和支架跳跃分别将肉桂酰胺和烟酰胺结构片段引入到吡唑-5-基-酰胺结构中,并合成了一系列衍生物。生物活性测定结果表明,大多数目标化合物对受试真菌表现出不同程度的抑制活性。值得注意的是,化合物G22、G28、G34、G38和G39对Valsa mali表现出优异的体外抗真菌活性,EC 50值分别为0.48、0.86、0.57、0.73和0.87 mg/L,并且该结果明显更有效。比啶酰菌胺(EC 50 = 2.80 mg/L) 更接近专业对照药物戊唑醇(EC 50 = 0.30 mg/L)。40 mg/L 的化合物G22和G34对苹果弧菌也表现出优异的体内保护和治疗效果。SEM和TEM观察表明化合物G22和G34可能影响正常的V. mali菌丝形态以及细胞超微结构。分子对接分析结果表明,G22和啶酰菌胺具有与SDH相似的结合模式,详细的SDH抑制实验验证了设计的化合物作为潜在SDH抑制剂的可行性。选择化合物G22和G3进行理论计算,该系列化合物的末端羧酸基团可能是影响抗真菌活性的关键区域。此外,对Apis mellifera l进行了毒性测试。结果表明,化合物G22和G34对意大利蜜蜂表现出低毒性。人口。上述结果表明,该系列吡唑-5-基酰胺衍生物作为潜在的低风险耐药农用SDHI杀菌剂具有开发前景。
更新日期:2023-11-03
中文翻译:

含有肉桂酰胺结构片段的吡唑-5-基-酰胺衍生物作为潜在琥珀酸脱氢酶抑制剂的发现
为了促进新型农用琥珀酸脱氢酶抑制剂(SDHI)杀菌剂的开发,我们通过碳链延伸和支架跳跃分别将肉桂酰胺和烟酰胺结构片段引入到吡唑-5-基-酰胺结构中,并合成了一系列衍生物。生物活性测定结果表明,大多数目标化合物对受试真菌表现出不同程度的抑制活性。值得注意的是,化合物G22、G28、G34、G38和G39对Valsa mali表现出优异的体外抗真菌活性,EC 50值分别为0.48、0.86、0.57、0.73和0.87 mg/L,并且该结果明显更有效。比啶酰菌胺(EC 50 = 2.80 mg/L) 更接近专业对照药物戊唑醇(EC 50 = 0.30 mg/L)。40 mg/L 的化合物G22和G34对苹果弧菌也表现出优异的体内保护和治疗效果。SEM和TEM观察表明化合物G22和G34可能影响正常的V. mali菌丝形态以及细胞超微结构。分子对接分析结果表明,G22和啶酰菌胺具有与SDH相似的结合模式,详细的SDH抑制实验验证了设计的化合物作为潜在SDH抑制剂的可行性。选择化合物G22和G3进行理论计算,该系列化合物的末端羧酸基团可能是影响抗真菌活性的关键区域。此外,对Apis mellifera l进行了毒性测试。结果表明,化合物G22和G34对意大利蜜蜂表现出低毒性。人口。上述结果表明,该系列吡唑-5-基酰胺衍生物作为潜在的低风险耐药农用SDHI杀菌剂具有开发前景。

































 京公网安备 11010802027423号
京公网安备 11010802027423号