当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Chiral Emissive Conjugated Corral for High-Affinity and Highly Enantioselective Recognition in Water
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-11-02 , DOI: 10.1002/anie.202315990 Rong Fu 1 , Qing-Yu Zhao 1 , Han Han 2 , Wen-Li Li 1 , Fang-Yuan Chen 3 , Chun Tang 2 , Wei Zhang 1 , Si-Dan Guo 3 , Dai-Yuan Li 3 , Wen-Chao Geng 3 , Dong-Sheng Guo 3 , Kang Cai 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-11-02 , DOI: 10.1002/anie.202315990 Rong Fu 1 , Qing-Yu Zhao 1 , Han Han 2 , Wen-Li Li 1 , Fang-Yuan Chen 3 , Chun Tang 2 , Wei Zhang 1 , Si-Dan Guo 3 , Dai-Yuan Li 3 , Wen-Chao Geng 3 , Dong-Sheng Guo 3 , Kang Cai 1
Affiliation
|
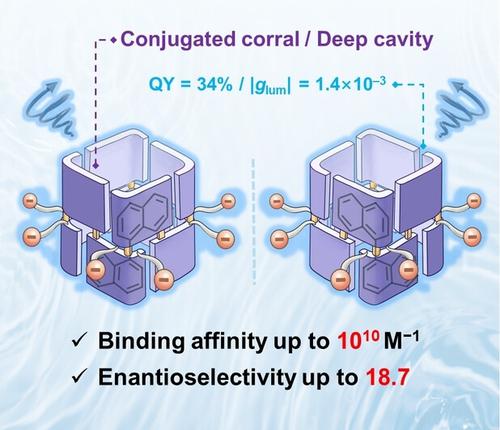
Accurately distinguishing between enantiomeric molecules is a fundamental challenge in the field of chemistry. However, there is still significant room for improvement in both the enantiomeric selectivity (KR(S)/KS(R)) and binding strength of most reported macrocyclic chiral receptors to meet the demands of practical application scenarios. Herein, we synthesized a water-soluble conjugated tubular host—namely, corral[4]BINOL—using a chiral 1,1′-bi-2-naphthol (BINOL) derivative as the repeating unit. The conjugated chiral backbone endows corral[4]BINOL with good fluorescent emission (QY=34 % ) and circularly polarized luminescence (|glum| up to 1.4×10−3) in water. Notably, corral[4]BINOL exhibits high recognition affinity up to 8.6×1010 M−1 towards achiral guests in water, and manifested excellent enantioselectivity up to 18.7 towards chiral substrates, both of which represent the highest values observed among chiral macrocycles in aqueous solution. The ultrastrong binding strength, outstanding enantioselectivity, and facile accessibility, together with the superior fluorescent and chiroptical properties, endow corral[4]BINOL with great potential for a wide range of applications.
中文翻译:

用于水中高亲和力和高对映选择性识别的手性发射共轭畜栏
准确区分对映体分子是化学领域的一个基本挑战。然而,大多数已报道的大环手性受体的对映体选择性(K R ( S ) /K S ( R ) )和结合强度仍有很大的改进空间,以满足实际应用场景的需求。在此,我们使用手性 1,1'-bi-2-naphthol (BINOL) 衍生物作为重复单元合成了一种水溶性共轭管状主体,即 corral[4]BINOL。共轭手性主链赋予corral[4]BINOL在水中良好的荧光发射(QY=34%)和圆偏振发光(| g lum | 高达1.4×10 -3 )。值得注意的是,corral[4]BINOL 对水中的非手性客体表现出高达 8.6×10 10 M -1的高识别亲和力,并且对手性底物表现出高达 18.7 的优异对映选择性,这两者都代表了水性手性大环化合物中观察到的最高值解决方案。超强的结合强度、出色的对映选择性和容易的可及性,以及优异的荧光和手性光学特性,赋予 Corral[4]BINOL 广泛应用的巨大潜力。
更新日期:2023-11-02
中文翻译:

用于水中高亲和力和高对映选择性识别的手性发射共轭畜栏
准确区分对映体分子是化学领域的一个基本挑战。然而,大多数已报道的大环手性受体的对映体选择性(K R ( S ) /K S ( R ) )和结合强度仍有很大的改进空间,以满足实际应用场景的需求。在此,我们使用手性 1,1'-bi-2-naphthol (BINOL) 衍生物作为重复单元合成了一种水溶性共轭管状主体,即 corral[4]BINOL。共轭手性主链赋予corral[4]BINOL在水中良好的荧光发射(QY=34%)和圆偏振发光(| g lum | 高达1.4×10 -3 )。值得注意的是,corral[4]BINOL 对水中的非手性客体表现出高达 8.6×10 10 M -1的高识别亲和力,并且对手性底物表现出高达 18.7 的优异对映选择性,这两者都代表了水性手性大环化合物中观察到的最高值解决方案。超强的结合强度、出色的对映选择性和容易的可及性,以及优异的荧光和手性光学特性,赋予 Corral[4]BINOL 广泛应用的巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号