当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Autocatalytic photoinduced oxidative dehydrogenation of pyrido[2,3-d]pyrimidin-7(8H)-ones: synthesis of C5–C6 unsaturated systems with concomitant formation of a long-lived radical
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-11-02 , DOI: 10.1039/d3qo01358h Claudi de Rocafiguera 1 , Vega Lloveras 2 , José Vidal-Gancedo 2 , Jordi Teixidó 1 , Roger Estrada-Tejedor 1 , José I. Borrell 1 , Raimon Puig de la Bellacasa 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-11-02 , DOI: 10.1039/d3qo01358h Claudi de Rocafiguera 1 , Vega Lloveras 2 , José Vidal-Gancedo 2 , Jordi Teixidó 1 , Roger Estrada-Tejedor 1 , José I. Borrell 1 , Raimon Puig de la Bellacasa 1
Affiliation
|
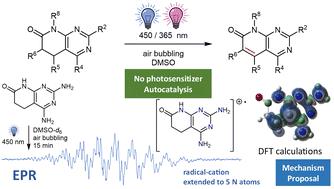
5,6-Dihydropyrido[2,3-d]pyrimidin-7(8H)-ones are readily accessed by a variety of methods and are a good scaffold for the development of biologically active compounds. However, they are very reluctant to dehydrogenate to give C5–C6 unsaturated compounds, usually with higher activity. A serendipitous discovery has allowed us to develop an autocatalytic photochemical dehydrogenation process by irradiating at 450 or 365 nm in DMSO, in the presence of air, and at room temperature the corresponding 5,6-dihydro derivative (with a variety of substituents at C2, C4, C5, C6, and N8) without adding any external photosensitizer. A complete study including reactions in DMSO-d6 followed by NMR spectroscopy, EPR experiments, the use of radical quenchers, spin-trapping techniques, and reaction with methyl viologen, complemented with ab initio calculations has allowed us to propose a mechanistic rationalization for such a process.
中文翻译:

吡啶并[2,3-d]嘧啶-7(8H)-酮的自催化光诱导氧化脱氢:C5-C6不饱和体系的合成,同时形成长寿命自由基
5,6-二氢吡啶并[2,3- d ]嘧啶-7(8 H )-酮很容易通过多种方法获得,并且是开发生物活性化合物的良好支架。然而,它们非常不愿意脱氢生成通常具有较高活性的C5-C6不饱和化合物。一个偶然的发现使我们能够开发出一种自催化光化学脱氢过程,通过在 DMSO 中、在空气存在下以及在室温下以 450 或 365 nm 的光照射相应的 5,6-二氢衍生物(在 C2 处具有多种取代基, C4、C5、C6 和 N8),无需添加任何外部光敏剂。一项完整的研究,包括 DMSO- d 6中的反应,然后是 NMR 波谱、EPR 实验、自由基猝灭剂的使用、自旋捕获技术以及与甲基紫精的反应,并辅以从头计算,使我们能够提出这种机制的合理化一个过程。
更新日期:2023-11-02
中文翻译:

吡啶并[2,3-d]嘧啶-7(8H)-酮的自催化光诱导氧化脱氢:C5-C6不饱和体系的合成,同时形成长寿命自由基
5,6-二氢吡啶并[2,3- d ]嘧啶-7(8 H )-酮很容易通过多种方法获得,并且是开发生物活性化合物的良好支架。然而,它们非常不愿意脱氢生成通常具有较高活性的C5-C6不饱和化合物。一个偶然的发现使我们能够开发出一种自催化光化学脱氢过程,通过在 DMSO 中、在空气存在下以及在室温下以 450 或 365 nm 的光照射相应的 5,6-二氢衍生物(在 C2 处具有多种取代基, C4、C5、C6 和 N8),无需添加任何外部光敏剂。一项完整的研究,包括 DMSO- d 6中的反应,然后是 NMR 波谱、EPR 实验、自由基猝灭剂的使用、自旋捕获技术以及与甲基紫精的反应,并辅以从头计算,使我们能够提出这种机制的合理化一个过程。































 京公网安备 11010802027423号
京公网安备 11010802027423号