当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt–Sulfur Coordination Chemistry Drives B12 Loading onto Methionine Synthase
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-02 , DOI: 10.1021/jacs.3c07941 Romila Mascarenhas 1 , Arkajit Guha 1 , Zhu Li 1 , Markus Ruetz 1 , Sojin An 1 , Javier Seravalli 2 , Ruma Banerjee 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-02 , DOI: 10.1021/jacs.3c07941 Romila Mascarenhas 1 , Arkajit Guha 1 , Zhu Li 1 , Markus Ruetz 1 , Sojin An 1 , Javier Seravalli 2 , Ruma Banerjee 1
Affiliation
|
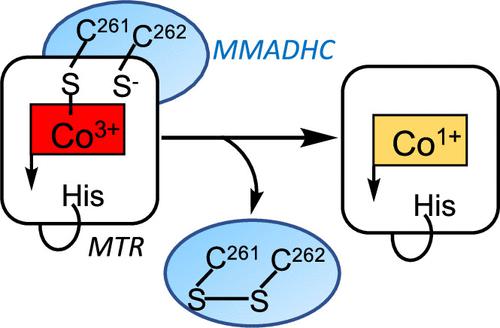
Cobalt–sulfur (Co–S) coordination is labile to both oxidation and reduction chemistry and is rarely seen in nature. Cobalamin (or vitamin B12) is an essential cobalt-containing organometallic cofactor in mammals and is escorted via an intricate network of chaperones to a single cytoplasmic target, methionine synthase. In this study, we report that the human cobalamin trafficking protein, MMADHC, exploits the chemical lability of Co–S coordination for cofactor off-loading onto methionine synthase. Cys-261 on MMADHC serves as the β-axial ligand to cobalamin. Complex formation between MMADHC and methionine synthase is signaled by loss of the lower axial nitrogen ligand, leading to five-coordinate thiolato-cobalamin. Nucleophilic displacement by the vicinal thiolate, Cys-262, completes cofactor transfer to methionine synthase and release of a cysteine disulfide-containing MMADHC. The physiological relevance of this mechanism is supported by clinical variants of MMADHC, which impair cofactor binding and off-loading, explaining the molecular basis of the associated homocystinuria.
中文翻译:

钴硫配位化学驱动 B12 装载到蛋氨酸合酶上
钴硫(Co-S)配位对氧化和还原化学都不稳定,在自然界中很少见。钴胺素(或维生素 B 12 )是哺乳动物中必需的含钴有机金属辅因子,并通过复杂的分子伴侣网络护送至单一细胞质靶标蛋氨酸合酶。在这项研究中,我们报告了人钴胺素运输蛋白 MMADHC 利用 Co-S 协调的化学不稳定性将辅因子卸载到蛋氨酸合酶上。 MMADHC 上的 Cys-261 作为钴胺素的 β 轴配体。 MMADHC 和甲硫氨酸合酶之间形成复合物的信号是下部轴向氮配体的丢失,从而产生五配位的硫醇钴胺素。邻位硫醇盐 Cys-262 的亲核置换完成了辅因子向蛋氨酸合酶的转移,并释放含有半胱氨酸二硫化物的 MMADHC。该机制的生理相关性得到 MMADHC 临床变异的支持,MMADHC 会损害辅因子结合和卸载,解释了相关同型半胱氨酸尿症的分子基础。
更新日期:2023-11-02
中文翻译:

钴硫配位化学驱动 B12 装载到蛋氨酸合酶上
钴硫(Co-S)配位对氧化和还原化学都不稳定,在自然界中很少见。钴胺素(或维生素 B 12 )是哺乳动物中必需的含钴有机金属辅因子,并通过复杂的分子伴侣网络护送至单一细胞质靶标蛋氨酸合酶。在这项研究中,我们报告了人钴胺素运输蛋白 MMADHC 利用 Co-S 协调的化学不稳定性将辅因子卸载到蛋氨酸合酶上。 MMADHC 上的 Cys-261 作为钴胺素的 β 轴配体。 MMADHC 和甲硫氨酸合酶之间形成复合物的信号是下部轴向氮配体的丢失,从而产生五配位的硫醇钴胺素。邻位硫醇盐 Cys-262 的亲核置换完成了辅因子向蛋氨酸合酶的转移,并释放含有半胱氨酸二硫化物的 MMADHC。该机制的生理相关性得到 MMADHC 临床变异的支持,MMADHC 会损害辅因子结合和卸载,解释了相关同型半胱氨酸尿症的分子基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号