Synlett ( IF 1.7 ) Pub Date : 2023-10-31 , DOI: 10.1055/a-2159-9400 Nurettin Menges 1, 2 , Volkan Tasdemir 3 , Hasan Genç 4
|
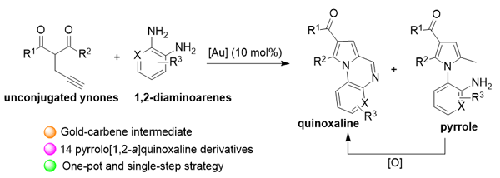
The pyrrolo[1,2-a]quinoxaline skeleton has significant potential for many biological and optical applications. Hence, in this study, unconjugated ynone derivatives were treated with 1,2-diaminoarenes in a gold-catalyzed cyclization to give 2-(1H-pyrrol-1-yl)anilines, which are valuable starting materials, and pyrrolo[1,2-a]quinoxalines by a one-pot and single-step approach. A reaction mechanism for the formation of the pyrrolo[1,2-a]quinoxaline skeleton featuring a key gold carbene intermediate is proposed. On the other hand, the methyl group on the C-2 position of the 2-(1H-pyrrol-1-yl)anilines was oxidized by SeO2 to give the pyrrolo[1,2-a]quinoxaline skeleton, resulting in 14 different pyrrolo[1,2-a]quinoxaline derivatives.
中文翻译:

通过金卡宾中间体合成 2-(1H-吡咯-1-基)苯胺和吡咯并[1,2-a]喹喔啉的黄金合成方法
吡咯并[1,2-a]喹喔啉骨架在许多生物和光学应用中具有巨大的潜力。因此,在本研究中,在金催化环化中用 1,2-二氨基芳烃处理非共轭炔酮衍生物,得到 2-(1 H-吡咯-1-基)苯胺(这是有价值的起始原料)和吡咯[1,通过一锅单步方法制备2- a ]喹喔啉。提出了以关键金卡宾中间体为特征的吡咯并[1,2- a ]喹喔啉骨架形成的反应机理。另一方面,2-(1H-吡咯-1-基)苯胺的C-2位甲基被SeO 2氧化,得到吡咯并[1,2- a ]喹喔啉骨架,得到14 种不同的吡咯并[1,2- a ]喹喔啉衍生物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号